Founded in 2008 in Quebec City by François-Thomas Michaud, Feldan Therapeutics is a biopharmaceutical company specializing in the intracellular delivery of therapeutic biomolecules. Its proprietary platform, the Feldan Shuttle, opens new possibilities for the treatment of skin and lung diseases by enabling precise targeting of diseased cells while sparing healthy ones. During the Effervescence Forum we were invited to a couple of weeks ago in Montreal, Canada, we sat with Mr. Michaud to know more about this disruptive technology.
A Disruptive Technology: The Feldan Shuttle
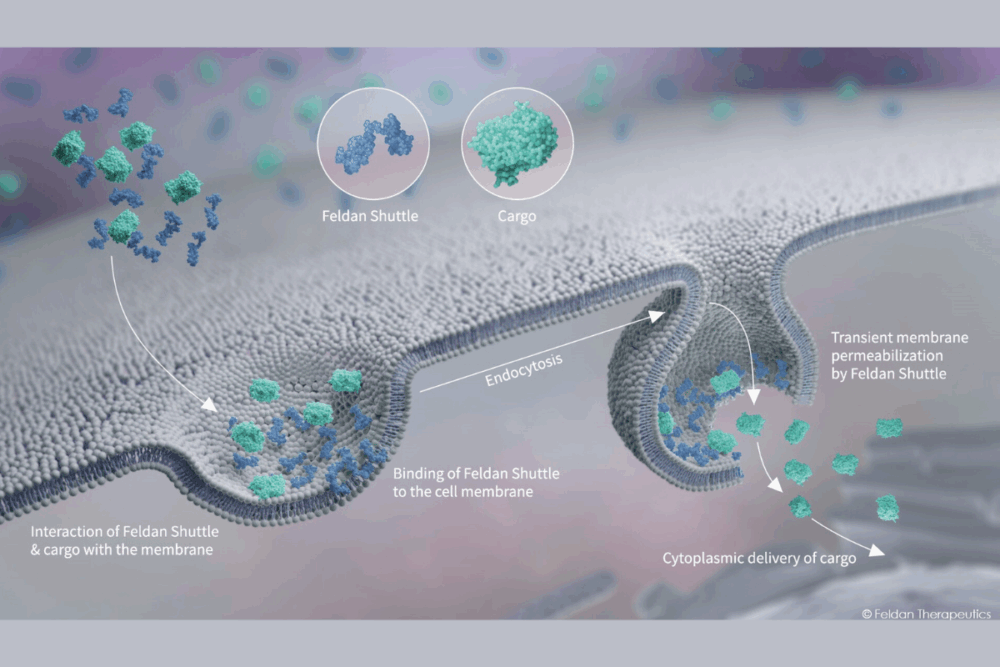
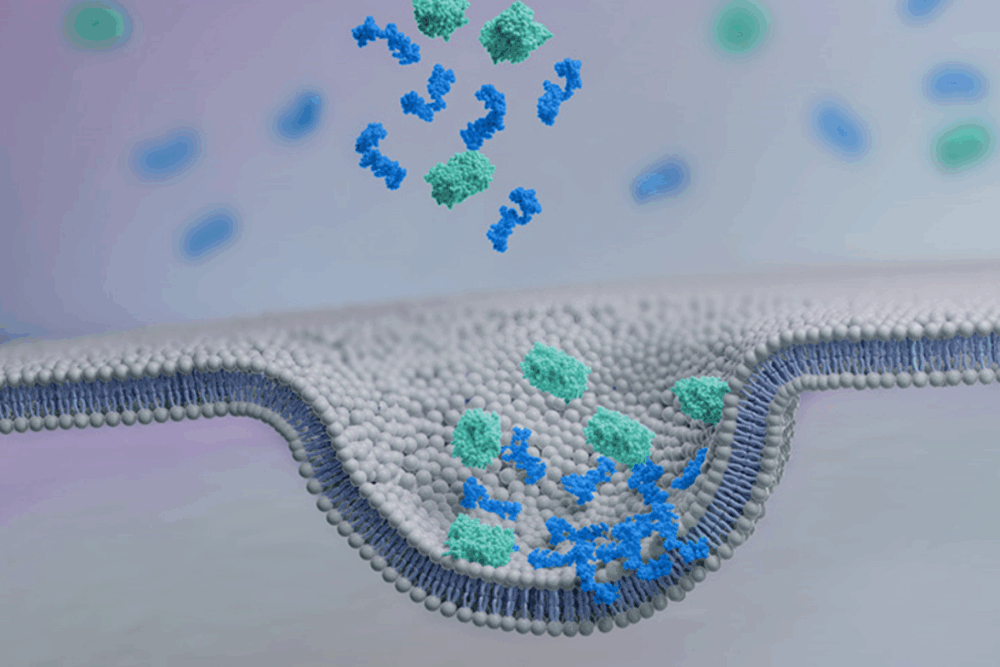
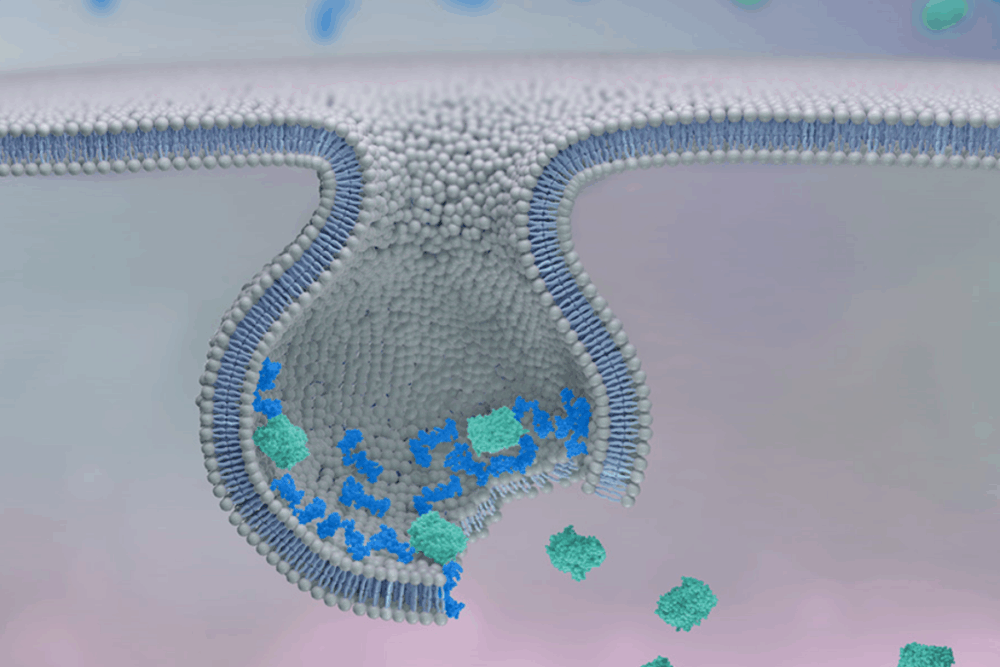
At the core of Feldan’s innovation lies the Feldan Shuttle, a peptide-based delivery system engineered to transport biomolecules—such as proteins, antibodies, or antisense oligonucleotides (ASOs)—directly into the cytoplasm of cells.
Unlike other delivery technologies, the Feldan Shuttle does not require covalent linkage between the peptide and the therapeutic cargo. Instead, simple mixing is sufficient, and natural chemical interactions ensure cellular entry. As Michaud explains:
“The hydrophobic components of the Shuttle bind to sugars on the cell surface, triggering endocytosis. However, the endosomes do not fully form, allowing the Shuttle to disrupt the membrane and release the molecules directly into the cytoplasm.”
This mechanism effectively circumvents one of the major limitations of traditional intracellular delivery methods: entrapment within endosomes. The Shuttle allows for safe, in vivo-compatible delivery without generating toxic byproducts.
“There are no size limitations per se. The smaller the molecule, the more efficient the delivery—but the system is capable of transporting a wide range of biomolecules, proteins, antibodies, or ASOs (antisense oligonucleotides).”
However, the technology is not suitable for mRNA or naked DNA, which are too highly charged to be effectively delivered by the Shuttle.
Localized Targeting, Precise Treatment
Feldan focuses its efforts on local delivery applications, primarily targeting the skin and lungs, with additional proof-of-concept work underway for ocular and auditory conditions. This strategy is driven both by the nature of the technology and strategic considerations related to clinical development.
“In recent years, our focus has shifted heavily toward ASO delivery. We’re able to inhibit specific proteins with high precision—particularly those inactive in healthy cells.”
This selectivity enables Feldan to pursue highly targeted treatments, including in certain cancers where cellular “patrolling” mechanisms are malfunctioning.
“The advantage is that these patrolling pathways are inactive in neighboring healthy cells. So even if those cells are exposed to the therapy, there is no effect.”
In the Clinic: Targeting Skin Cancer
One of Feldan’s flagship programs, FLD-103, targets basal cell carcinoma (BCC)—the most common form of skin cancer. The treatment involves local injection of a Shuttle-delivered ASO that neutralizes a protein reactivated by sun-induced genetic mutations.
“By doing this, we effectively shut down the defective pathway, and the cancerous cells undergo apoptosis.”
A Phase 1/2 clinical trial is currently underway in Australia. The trial features a dose-escalation phase followed by a placebo-controlled expansion. All patients eventually undergo surgical removal of the tumor, following standard of care.
“We’re currently three-quarters of the way through the dose-escalation phase. We’re testing one to four injections in patients aged 18 to 90.”
This trial model enables an efficient evaluation of safety and efficacy at a relatively low cost, while paving the way for more complex future indications.
A Promising Pipeline in Pulmonary Disease
Feldan is also advancing a preclinical program targeting pulmonary conditions, particularly those characterized by excess mucus production and bronchial dilation. The Shuttle has demonstrated efficient ASO delivery to the lungs in porcine and primate models.
“Our goal is to reduce mucus levels. We’ve dosed a significant number of animals and are now assembling the preclinical package and financing.”
Although this represents a larger therapeutic market, it comes with greater regulatory and financial challenges.
And What About CRISPR?
Feldan previously collaborated with the Somatic Cell Gene Editing consortium on pulmonary CRISPR delivery programs. However, the company ultimately pivoted away from genome editing, citing constraints in intellectual property and funding.
“We did some work in that space, but pursuing it independently was not sustainable. The main issue was the lack of IP rights on the molecules themselves. For rare diseases with only 300 to 400 patients worldwide, it’s extremely difficult to secure funding.”
Today, Feldan focuses on more common indications where its platform can deliver quicker and more tangible results.
Looking Ahead: Clinical Development and Fundraising
Feldan plans to complete its FLD-103 clinical trial by the end of 2026 and aims to initiate a clinical program for lung disease within the same timeframe. A third preclinical program, targeting a more aggressive type of skin cancer, is also underway.
“Our ideal scenario is to have one program in the clinic, one preparing to enter, and a third in preclinical development.”
To support these goals, the company is actively seeking additional funding.
Strategic Partnerships and Financial Support
Feldan recently secured $21 million in Series B financing, co-led by Genesys Capital and the Fonds de solidarité FTQ, with participation from Linearis Ventures and other investors. These funds will accelerate the clinical development of FLD-103 and expand the company’s therapeutic pipeline.
Additionally, Feldan collaborates with Amgen Canada and the CQDM to optimize its pulmonary delivery platform. This partnership seeks to develop new treatments for debilitating respiratory diseases by overcoming the natural barriers of the airways that often limit therapeutic efficacy.
To learn more, explore our other articles on Quebec-based innovations following our coverage of the Effervescence forum: