Chinese company Asclepius Meditec presented its novel hydrogyn-oxygen inhalation platform during the Canton Fair in China last October. The company announced a global reach currently underway.
Asclepius Meditec’s award-winning medical-grade hydrogen-oxygen nebulizer received global market approval in 2020 and was listed as the only designated equipment in China’s COVID-19 treatment protocol. Already approved in China as a Class III medical device, the system is now positioned by its developer for future regulatory pathways in Europe and the United States.
During the Canton Fair in China, we spoke directly with company representatives and learned more about international ambitions for its hydrogen–oxygen inhalation technology. Important advancements are scheduled for this year.
As a Chinese medical technology company founded in the early 2010s, Asclepius Meditec is best known for developing what it describes as the world’s first medical-grade hydrogen–oxygen nebulizer. The device delivers a controlled mixture of hydrogen and oxygen gases and has been studied primarily in respiratory and critical care contexts. According to the company, preparations are underway to pursue EU conformity under the Medical Device Regulation (MDR) framework and to explore a De Novo classification pathway with the U.S. Food and Drug Administration (FDA).
A Hydrogen–Oxygen Inhalation System with Class III Status in China
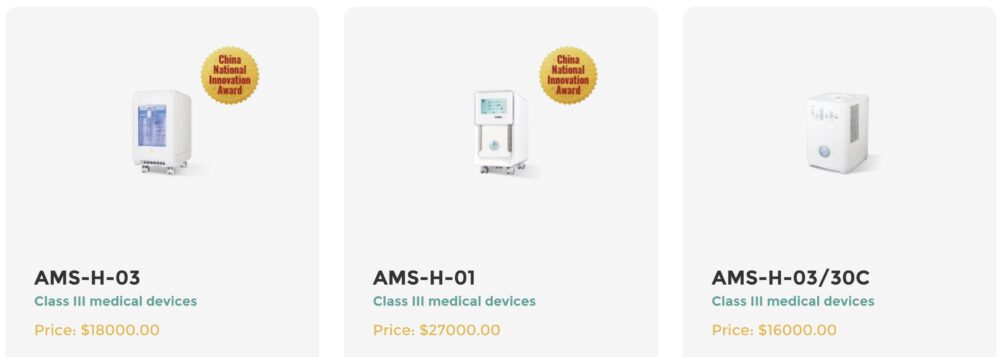
The Asclepius hydrogen–oxygen inhaler (commercialized in China under models such as the AMS-H series) is regulated domestically as a Class III medical device, the highest risk category under China’s National Medical Products Administration (NMPA). This designation reflects the intended clinical use and the novelty of the therapeutic approach.
The system generates hydrogen and oxygen through water electrolysis, delivering a fixed gas mixture—commonly cited as approximately 66% hydrogen and 33% oxygen—via inhalation. Unlike conventional oxygen therapy, the device is positioned as a combined respiratory support and adjunctive therapeutic system, rather than a simple oxygen concentrator.
In 2020, the device received national-level regulatory recognition in China and was referenced in Chinese clinical guidance during the COVID-19 public health emergency. According to company statements, it was listed as a designated supportive therapy option within certain diagnostic and treatment protocols, an inclusion that significantly increased clinical exposure and real-world use.
Clinical Rationale: Molecular Hydrogen in Respiratory Care
Interest in molecular hydrogen (H₂) as a therapeutic gas predates the COVID-19 pandemic. Over the past two decades, hydrogen has been investigated for its selective antioxidant and anti-inflammatory properties, with preclinical and clinical studies exploring potential benefits in ischemia–reperfusion injury, sepsis, and pulmonary inflammation.
In the respiratory setting, hydrogen inhalation has been studied as a means to mitigate oxidative stress and inflammatory cascades associated with acute lung injury. During the COVID-19 pandemic, this rationale gained renewed attention, particularly for patients experiencing dyspnea and hypoxemia despite conventional oxygen therapy.
Clinical studies conducted in China, including randomized and controlled investigations registered in international trial databases, evaluated hydrogen–oxygen inhalation in patients with COVID-19–related respiratory symptoms. Reported outcomes focused on symptom relief—such as reductions in dyspnea and improvements in respiratory comfort—rather than direct antiviral effects. Importantly for clinicians, these studies generally characterized the intervention as well-tolerated when used under controlled conditions.
While COVID-19 accelerated awareness of hydrogen–oxygen inhalation, Asclepius Meditec positions the technology as relevant beyond infectious disease. The company has indicated interest in applications across chronic respiratory conditions, rehabilitation medicine, and supportive care, where modulation of inflammation and oxidative stress may offer adjunctive benefits. At present, the strongest body of human data remains concentrated in acute respiratory illness.
European Regulatory Pathway: MDR Compliance in Progress
According to information shared by Asclepius Meditec, the company is preparing for entry into the European market under the EU Medical Device Regulation (MDR). This would require conformity assessment by a notified body, demonstration of clinical performance and safety, and full technical documentation in line with MDR requirements.
No publicly accessible EU CE certificate has yet been identified for the hydrogen–oxygen inhaler. Company representatives state that conformity testing and documentation processes are underway, with CE marking targeted in the coming years. For medical professionals and procurement specialists in Europe, this means the device should currently be regarded as not yet authorized for routine clinical use within the EU.
The MDR pathway may prove particularly demanding given the device’s novel mechanism and gas composition, which may place it in higher-risk categories and require robust clinical evidence.
Exploring a De Novo Pathway in the United States
Asclepius Meditec has also indicated its intention to explore regulatory entry into the U.S. market via the FDA’s De Novo classification process. This pathway is typically used for first-of-a-kind devices that present low to moderate risk but lack a suitable predicate device for 510(k) clearance.
A successful De Novo submission would establish a new device classification and special controls, potentially opening the door for future devices in the same category. However, at the time of writing, there is no publicly listed De Novo submission or FDA decision associated with Asclepius Meditec’s hydrogen–oxygen inhaler.
For U.S. clinicians, the technology should therefore be considered investigational until formal FDA authorization is granted.
Asclepius Meditec’s Class III approval and pandemic-era use in China underscore both regulatory confidence at the national level and real-world clinical exposure. At the same time, international expansion will depend on rigorous regulatory review and the generation of evidence aligned with European and U.S. standards. For medical professionals following developments in respiratory and critical care technologies, the coming years will be pivotal in determining whether hydrogen–oxygen inhalation transitions from a regionally adopted innovation to a globally recognized therapeutic modality.