Can a small device under your mattress change the way we understand — and treat — sleep disorders? According to Mathilde Chevalier-Pruvo, Director of Health Vision and Public Affairs at Withings, the answer is yes. In this conversation, she explains how real-world data collected via smart sensors is revolutionizing sleep research, enabling earlier diagnoses of conditions like sleep apnea, hypertension, and even cognitive decline.
French company Withings designs, develops, and markets connected devices such as smartwatches, scales and sleep analyzers. With their Sleep Analyzer, they aim to analyze the relationship between sleep and health in collaboration with a sleep research center in Australia. The Withings Sleep Analyzer is a sensor placed under the mattress. Once it’s in place, the user doesn’t need to do anything and the device will start collecting data on patients with sleep disorders.
How is this transforming sleep research?
Mathilde Chevalier-Pruvo: “It’s all about real-world data. Traditional sleep studies happen in hospitals, where the environment — noisy corridors, electrodes, unfamiliar beds — is far from ideal. Polysomnography is expensive and limited to one or two nights. Our solution costs about €200 and can collect automatic, passive data over several nights at home. This allows for a much clearer picture of how sleep disorders fluctuate, night by night.”

What kind of insights are you getting that weren’t possible before?
Mathilde Chevalier-Pruvo: “We’ve run several studies now — four already published. Because the sensor records over many nights, and under real-life conditions, we’ve been able to see how factors like late meals or screen time impact sleep. This is key because conditions like insomnia and sleep apnea vary depending on daily habits. Our system also collects respiratory rate, heart rate, and even allows users to complete questionnaires for additional context.”
What scale are you working at now?
Mathilde Chevalier-Pruvo: “That’s one of the most revolutionary aspects. Traditional studies might include a few dozen participants. With our system, we’re talking cohorts of over 10,000 people — sometimes tracked over six months. Researchers tell us it’s a game-changer.”
Do researchers buy these devices or are they provided?
Mathilde Chevalier-Pruvo: “It depends. With the Australian team, we have a strong partnership — we provide the devices. Other research groups purchase them.”
And then, what kind of studies are being done with the data?
Mathilde Chevalier-Pruvo: “They’re both prospective and retrospective. Withings has been around for 15 years, so we have anonymized, GDPR-compliant data going back that far — covering 50 different biomarkers. Researchers are using this data to explore links between sleep disorders and conditions like hypertension. We were actually the first to show that snoring alone increases hypertension risk, simply because we were measuring both.”
What’s in it for Withings to share this data?
Mathilde Chevalier-Pruvo: “It’s about public benefit. We don’t charge for access — it’s always under strict legal agreements. And we benefit too: when researchers find new health correlations, we can build those into our monitoring tools.”

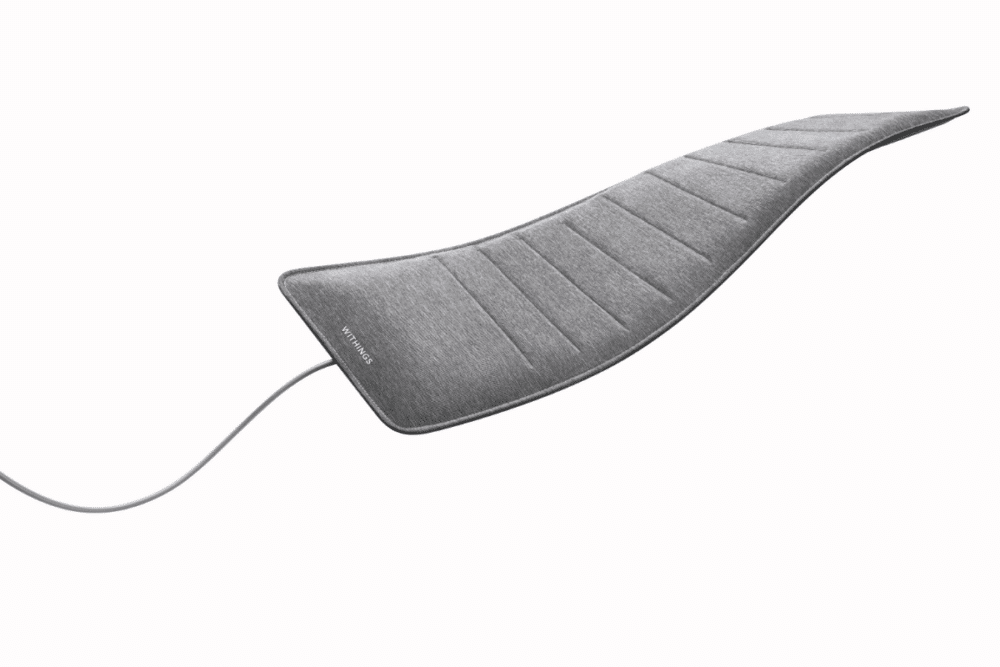

How many nights of sleep data are needed to identify trends?
Mathilde Chevalier-Pruvo: “Our first study with the Australian researchers showed it takes at least 14 nights. Sleep apnea, for example, can vary based on so many things — late eating, activity levels, weight. A single-night study like in hospitals has a 50% error rate: 30% false negatives and 20% false positives.”
How accurate is your device?
Mathilde Chevalier-Pruvo: “We have a 94% accuracy rate over a 14-night period. That’s a 7% margin of error — significantly better than traditional methods.”
Once the data is collected, how is it analyzed?
Mathilde Chevalier-Pruvo: “We use our own AI tools to find correlations and develop scoring systems — like our sleep apnea score. Our research partners also have their own data science teams using AI to uncover patterns, like the snoring–hypertension link.”
Are you uncovering trends or certainties?
Mathilde Chevalier-Pruvo: “We’ve gone beyond trends. We’ve proven a direct connection between sleep apnea and cardiovascular problems, like hypertension and heart failure, which in turn reduce life expectancy. We’ve also demonstrated that irregular sleep patterns — both in duration and bedtime — significantly increase cardiovascular risk.”
Are there any new clinical applications of your technology?
Mathilde Chevalier-Pruvo: “Yes, for example, we’re now rolling out Sleep Analyzers in diabetology departments in French hospitals. Sleep apnea is common in people with diabetes. This allows automatic detection during their overnight hospital stays — even if it’s just one night, because we already have correlations between diabetes and apnea.”
What’s the long-term goal here?
Mathilde Chevalier-Pruvo: “To enable early diagnosis through connected devices. Right now, it can take up to two years to get a sleep study in France. That’s unacceptable. If sleep apnea is diagnosed early, it’s treatable. If not, it can reduce life expectancy by up to 12 years. We’re also collaborating with institutions like the Imperial College in London to study how sleep disorders may even contribute to Alzheimer’s.”
Could connected devices replace medical consultations?
Mathilde Chevalier-Pruvo: “Not consultations — but diagnostic testing, yes. In the U.S., our Sleep Analyzer is FDA-approved. Doctors prescribe it, insurance reimburses it, and they use the data to diagnose patients remotely. We’d like to see the same thing happen in France.”
What’s holding things back in France?
Mathilde Chevalier-Pruvo: “Doctors are on board, and hospitals are starting to adopt it. But the bottleneck is at the reimbursement and policy level. Chronic diseases already make up 60% of health spending and are growing fast. We need public authorities to move faster.”