Born out of the CHUM Research Center and the Université de Montréal, Targa Biomedical is a Montreal-based spin-off revolutionizing both organ transplantation and regenerative medicine. Co-founded in 2018 by Dr. Shant Der Sarkissian and Dr. Nicolas Noiseux, Targa is developing advanced biopharmaceutical solutions that address two major challenges in the healthcare sector: the global shortage of transplantable organs and the fragility of cellular therapies. As the company prepares for its first clinical trials in humans, its dual technological platforms are gaining attention in both academic and industry circles for their potential to reshape standards of care.
During the Effervescence Forum we were invited to at the end of April (read our story on Quebec’s Life Sciences Sector), we met with Dr. Shant Der Sarkissian, co-founder and CEO of Targa Biomedical to learn more about how their technology aims to open a new era in organ transplantation and cell therapy.
Expanding the Pool: Making Marginal Organs Transplantable
At the heart of Targa’s innovation lies a bold mission: to salvage organs previously deemed unfit for transplantation. Today, most transplantable organs come from neurologically deceased donors, or donation after brain death (DBD) with heartbeats still intact. However, a significant number of potential donations exist among donors after circulatory death (DCD), a category largely untapped due to the potential of ischemic damage that occurs during the dying and procurement process. Roughly, only 10 to 15% of DCD lungs are typically used today.
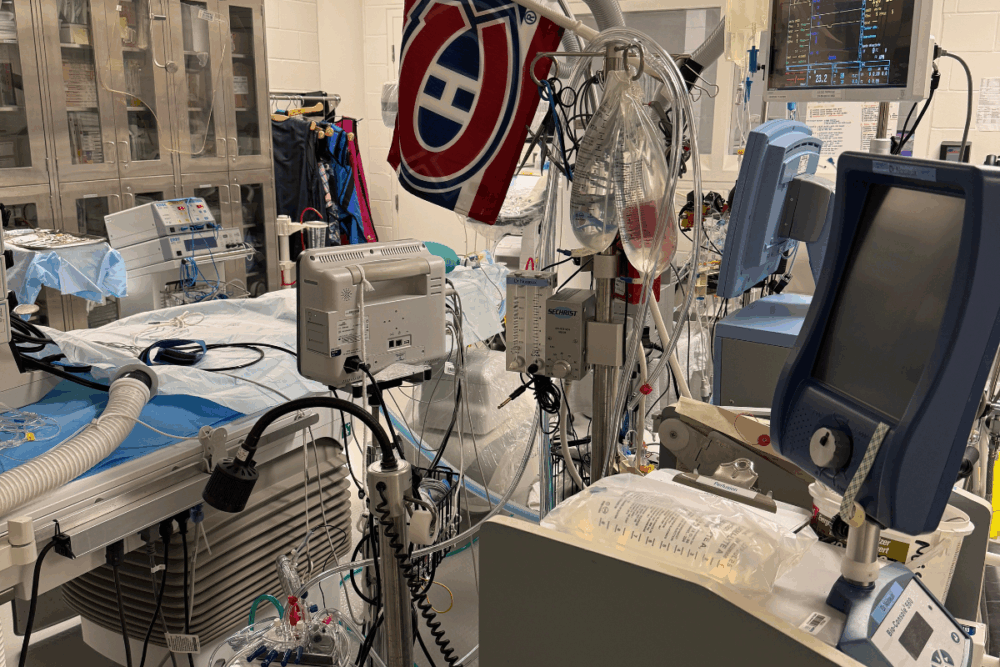
Therefore, Targa developed a proprietary solution designed to minimize ischemic damage and oxidative stress. Used during cold storage and ex vivo organ perfusion, the solution aims to rescue and repair damage caused by hypoxia and reperfusion.
Pharmacological Precision: How It Works
“When circulation stops, tissues suffer from a lack of oxygen. But when it restarts, reperfusion generates free radicals, which further damage the tissue,” Der Sarkissian explains. “Our solution creates a protective halo that can last for several days after treatment, buying critical time and preserving function.”
Dr. Der Sarkissian explains the process:
“We perfuse the organ outside the body with our solution for a few hours. Once markers and functional results meet transplant thresholds, we deem the organ suitable for transplantation.”
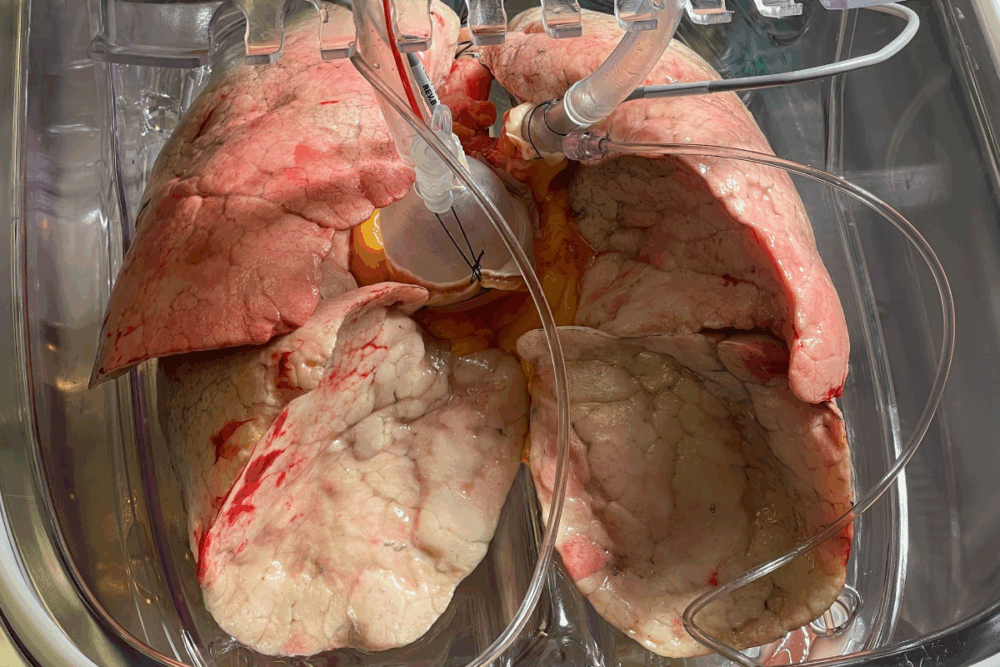
This approach has already been tested successfully on lungs, livers, and hearts in both small and large animal models
“We’re seeing very encouraging results—improvement of oxygen diffusion lung function, metabolic biomarkers, and bronchoscopy data, all validate organ quality and functionality after treatment,” he adds.
The company’s solution is also effective at varying temperatures, including 4°C—standard for cold organ preservation. This makes it adaptable across the entire chain of custody, from procurement to transplantation.

Montreal First: Toward Human Trials in 2025
Targa’s first-in-human clinical trial is slated for 2025. All preclinical research has been completed, and the company is ready for Good Manufacturing Practice (GMP) production phase.
“Montreal is our home base,” says Dr. Der Sarkissian. “It’s only fitting to start in Montreal. We’ve already secured half the funding we need, and we’re on track to treat our first patient this year.”
The team has built robust clinical collaborations with transplant surgeons across pulmonary, hepatic, and cardiac specialties. The infrastructure is in place, but access to high-end ex vivo perfusion systems—offered by industry players like TransMedics, XVIVO, OrganOx, and Bridge to Life—remains a barrier for many institutions.
“Not every hospital has the equipment or trained personnel for ex vivo perfusion,” he notes. “It’s a rapidly expanding field, and we’re arriving just in time with a transformative solution to further expand access.”
Beyond Transplantation: Enhancing Cell Therapy Pipelines
Targa’s second technological vertical targets the manufacturing processes behind regenerative medicine. The company is developing solutions that improve both hypothermic preservation and cryopreservation of therapeutic cells—a growing need as cell therapy pipelines expand globally.
“Whether it’s transporting cells refrigerated or at ambient temperature, or storing them in cryogenic conditions, preserving their viability and function is key. Our solutions can dramatically improve quality and outcomes.”
The technology has already demonstrated promise in real-world scenarios, with several collaborations with biotech companies.
“These molecules work across different cell types, and we’re looking at how they can also be integrated into workflows involving 3D bioprinting and bio-gels,” says Der Sarkissian. “The aim is simple: boost cellular viability, no matter the stress the cells are subjected to.”
Scaling Impact: From Quebec to Global Markets
While Targa’s roots are firmly in Montreal, its ambitions are global. The company is already in discussions with U.S. hospital centers and views the American market as a key next step. Europe is also a target of high interest to expand Targa’s reach and impact across international markets.
“Our biggest challenge right now is identifying the right partners—clinical and industrial—who can help us scale this vision,” Der Sarkissian admits. “The technology is sound, and the preclinical data is extremely promising. Now it’s a matter of execution.”
With organ shortages a persistent problem worldwide, and demand for regenerative therapies on the rise, Targa Biomedical is well positioned to become a transformative player in the life sciences sector.