Over the past 20 years, COMEN’s monitoring product line has evolved from traditional monitors, multi-parameter monitors and modular monitors to specialized monitors. It has now developed into a fifth-generation life information platform.
Starting from May 26, 2021, the previous EU Directive on medical devices (93/42/EEC) has been replaced by Regulation MDR (EU) 2017/745. This regulation lays out the necessary requirements and procedures for assessing conformity that must be followed before medical devices can be introduced into the European Economic Area.
If a medical device belongs to class IIa, IIb, or III, then working with a Notified Body is necessary.
Key Changes of the MDR
The MDR is significantly different from the previous EU directives in several ways regarding its content. The scope of the application has been expanded, and there are more specific requirements for the creation and maintenance of technical documentation.
The clinical evaluation requirements have become stricter and a new control procedure for high-risk medical devices has been introduced, known as the “scrutiny procedure.” The MDR also requires a designated person responsible for regulatory compliance and tighter requirements for post-market surveillance of medical devices and the vigilance system.
The introduction of a unique product identification number (UDI system) improves the identification and traceability of devices. The MDR promotes transparency by introducing a central European database (EUDAMED) and obligating manufacturers to provide coverage in the event of liability.
The MDR also introduces a new group of reusable surgical instruments and new classification rules, including for software, devices containing nanomaterials and medical devices with material components.
The new regulation aims to establish a consistent and standardized system for designating and monitoring notified bodies across Europe, based on more specific and rigorous requirements. As a result of these increased requirements, the number of notified bodies that can fit and can issue the MDR certificate in Europe has already decreased in recent years, and further consolidation is expected in the future.
The major areas of change in the MDR include:
- Technical Documentation
- Requirements for clinical evaluation and post-market clinical follow-up
- Increased traceability of devices (UDI)
COMEN’s Patient Monitors
Comen’s patient monitoring products have recently obtained the CE certificate under Medical Directive Regulations (MDR). This accomplishment is a testament to our company’s commitment to providing high-quality and safe medical devices that meet the most rigorous standards.
We take pride in our reputation for excellence in the industry and our ongoing efforts to stay at the forefront of innovation in patient monitoring technology. With our MDR-compliant products, healthcare professionals and patients alike can trust in the accuracy, reliability, and safety of our devices to support better health outcomes.
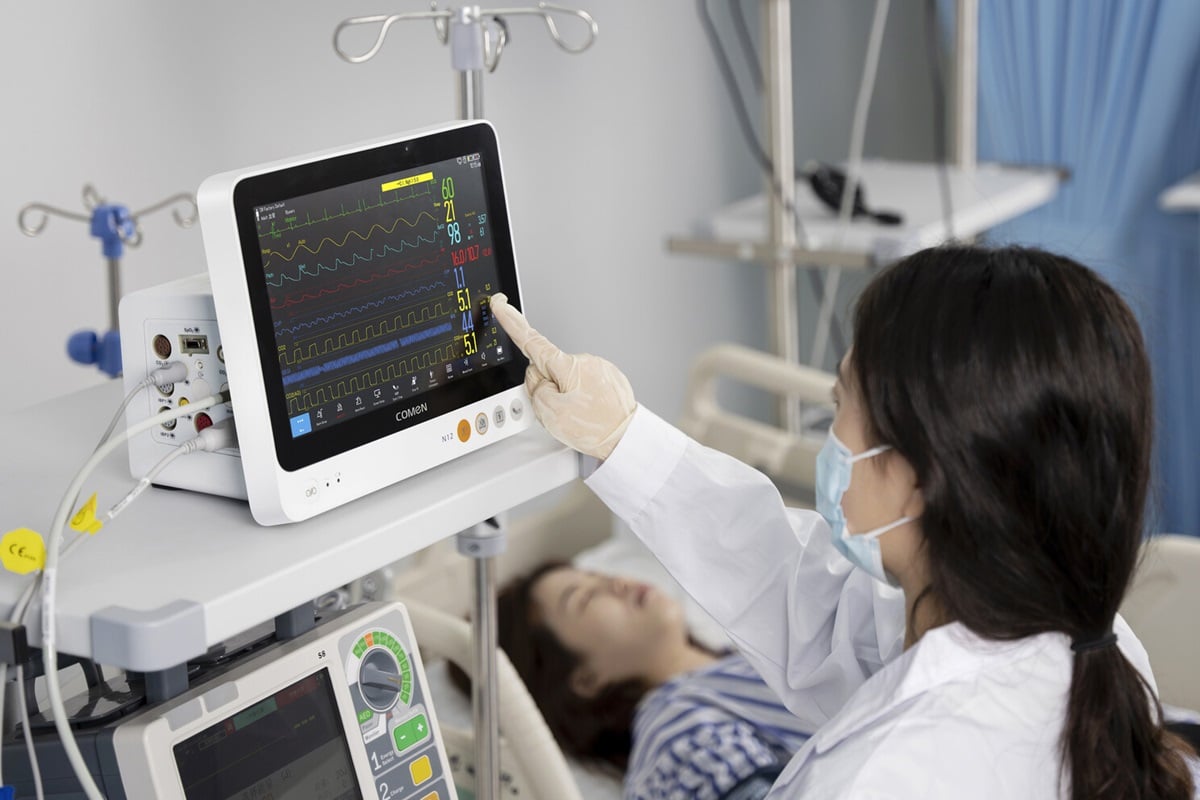
The patient monitor production line of COMEN has received recognition for its quality and efficiency. Started with monitoring devices and has been involved in this industry for 20 years since 2002. In 2009, we developed the world’s first dedicated neonatal monitor and subsequently developed specialized monitors for cardiovascular, obstetrics, emergency transportation, PICC and other fields.
These products are tailored to meet the clinical needs of various departments and are still unique in the market today. COMEN’s concept of specialized monitoring has created a new trend in the upgrading of the monitoring industry.
Our new K-series and N-series monitors are life information support platforms that not only provide physiological monitoring but also integrate equipment such as ventilators and ultrasound imaging on this platform for centralized display, intelligent analysis, and comprehensive support for clinical decision-making.