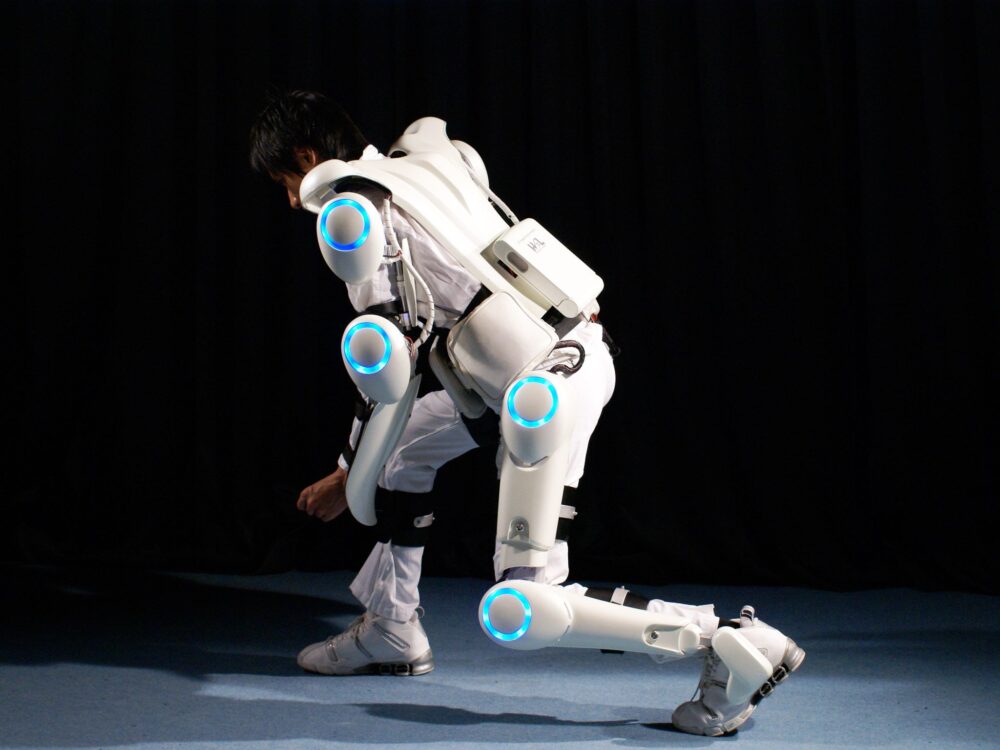
At RehaCare, Cyberdyne Care Robotics GmbH presented its new “for child” version of HAL, currently ready for worldwide distribution.
Düsseldorf, RehaCare 2025 — Japanese firm Cyberdyne Care Robotics GmbH today confirmed that its “for child” or small-size version of HAL (Hybrid Assistive Limb) will be made available globally this year, including in Germany and across Europe.
The small-size HAL, formally named Medical HAL Lower Limb Type B, has already been approved in Japan, the US, and the EU, opening new possibilities for pediatric rehabilitation. We spoke with Read Abo Facher, Sales Manager for Cyberdyne Care Robotics, at the opening of RehaCare to understand the significance of this rollout.
In Germany? Visit the Cyberdyne Care Robotics GmbH Europe headquarter office. Their office is behind BG Universitätsklinikum Bergmannsheil Bochum.
Behind the Technology of HAL and Its “For Child” Version
HAL is a wearable, neurologically-controlled assistive exoskeleton designed to support and rehabilitate impaired motor function by detecting subtle bio-electrical signals (BES) from muscles. These signals are those that would otherwise be lost or discarded due to injury or disease, and HAL amplifies them via sensors to drive motion in the lower limbs. It is meant for rehabilitation, not continuous long-term wear. As the representative told us:
“It’s not for long-term use, it’s like we say like 30 to 60 sessions, it depends on the patient and the situation that has.”
The “for child” / small-size HAL targets patients measuring 100 to 150 cm in height, filling a gap where previous HAL devices required a larger body frame to operate safely and effectively. In Japan, the device was approved on January 31, 2025, by the Japanese Minister of Health, Labour and Welfare.
In the US, the FDA cleared both the small model and an expanded indication list (including cerebral palsy) on May 7, 2024. The EU gave conformity certification (MDR) to the small lower limb version on December 12, 2024, allowing CE-marking and sale across member states.
Read Abo Facher noted that prior to this, many smaller children were unable to undergo Cybernics Treatment because of height/size constraints. The new model extends access to neuromuscular patients, children with cerebral palsy, hereditary spastic paraplegia, and other conditions, but still intended as a therapeutic intervention over a defined course rather than permanent assistive wear.


Understanding the Difference between BES and BCIs, and the Foundation in Cybernics
A crucial technical distinction lies between bio-electrical signals (BES) and brain-computer interfaces (BCIs). BES are the electrical signals generated in muscles (or nerves en route to muscles) in response to voluntary intention, which may persist even when motion is compromised.
HAL detects these via surface sensors: signals that would otherwise go unused—lost because the brain-muscle pathway is disrupted or weakened. In contrast, BCIs typically measure brain activity directly (via EEG, implanted electrodes, etc.) to infer intent, often bypassing peripheral nerves/muscles. BES thus are more specific to individual muscle groups and movement attempts; BCIs can suffer from noise, interference, weaker site specificity, and latency issues.
The theory and practice of HAL draw deeply on the work of Prof. Dr. Yoshiyuki Sankai of Tsukuba University, who has led cybernics research since around 1987. His early work concerned “interactive BioFeedback” (iBF) theory, combining human intention, robotics, sensors, and real-time feedback to recover or enhance motor function. Over decades, this has led from laboratory experiments to clinical trials and regulatory approval. The small model HAL is the latest instantiation of that lineage.


Cybernics and Roll-out of the Small-size HAL
From the late 1980s, Prof. Sankai’s lab in Tsukuba pioneered the core theoretical models: how to detect intention signals, map them into assistive motion, and create closed-loop feedback so that the nervous system can adapt (neuroplasticity). Over time, prototypes in the 1990s evolved into commercial HAL devices in the 2000s, regulatory approvals in the 2010s, and widespread use in medical institutions worldwide.
While the roll-out of small-size HAL is promising, there are challenges. Rehabilitation clinics will need to train physiotherapists and clinicians in HAL’s use. It is critical to monitor for fatigue, skin safety around sensors, and the careful calibration of assistance versus patient effort to maximize neuroplastic gains and avoid overreliance.
The company emphasizes collaboration with universities and medical centers to generate data. As Prof. Sankai once put it in a published interview:
“HAL is not just a mechanical assist: it’s a medium for restoring voluntary motor control.”
The global release in 2025 of the small-size HAL marks a milestone. Children who were previously unable to benefit because of size limitations now have access to regenerative technology powered by BES and rooted in decades of cybernics research. For medical professionals, this opens new clinical pathways in pediatric rehabilitation: improved mobility and potentially long-lasting improvements. As always, outcomes will depend on careful patient selection, session planning, and monitoring—but the horizon has broadened.