Treating neurological and psychological disorders remains a significant challenge in modern medicine. The pathogenesis of such diseases is complex. Conventional pharmacological interventions can be effective in treating specific symptoms, but they mostly fail to address the complexity of the diseases and consequently also fail in treating or delaying their progression. In this context, transcranial photobiomodulation therapy (tPBM) is gaining increasing interest as it is a broad-acting approach that can in many cases help our brains restore or improve crucial natural processes at the core of neurological and mental health. It thereby allows therapists to treat disorders as a whole system.
Transcranial photobiomodulation therapy relies on the application of near-infrared light to the brain, where it is absorbed by photoreceptors. It is non-invasive, non-thermal, painless and, if applied correctly, completely free of side effects. It is applied directly on the head and penetrates through the skin, cranium and all the other tissue to eventually reach the brain. The ability of infrared photons to reach the brain tissue has been shown in different simulations and studies. The deepest penetrating photons can reach a depth of up to 5cm.
Over the last years, solid scientific data on the therapy´s application in the following areas has been published: neurodegenerative diseases, psychological disorders, brain injuries, headaches, improved cognitive function in healthy individuals and neurodevelopmental disorders such as Autism.
The absorption process leads to different primary and secondary beneficial effects. The most important effects are:
1) More overall energy due to increased Adenosine Triphosphate production
Adenosine Triphosphate (ATP) is the energy source used on a cellular level and thus often called the cellular “energy currency.” It is consumed in almost all basic physiological processes. Its synthesis occurs in our mitochondria in three steps called a) glycolysis,
b) tricarboxylic acid cycle, and c) oxidative phosphorylation.
tPBM acts on the last step, oxidative phosphorylation, where ATP is produced through electron transfer processes in four different complexes. The last complex is an enzyme called Cytochrome C Oxydase (CCO). There, copper reduces oxygen to water and ATP is produced in the process.
What is essential in our context is that CCO can absorb red and infrared light photons. The absorbed energy accelerates the electron transfer processes, making more electrons available in CCO, consequently leading to an increase in ATP output directly following the intervention.


In addition to the immediate increase in ATP output, there are also long-term effects: when mitochondria sense that more energy is available, they signal this information to the nucleus. This alters the gene expression to enhance mitochondrial function and form new mitochondria. The process is called retrograde mitochondrial signaling.
Keeping in mind that the brain is the organ with the highest energy consumption and that it has the second highest mitochondria density of all our organs (after the heart), it is particularly vulnerable to disruptions of its energy sources. Most neurodegenerative diseases are characterized by mitochondrial dysfunction, a loss of mitochondrial membrane potential, and depletion of ATP. Interventions that can improve ATP production and thus improve brain metabolism – such as tPBM – can therefore play a big part in a comprehensive strategy for improving cognitive function and possibly slowing or preventing cognitive decline.
2) Increased cerebral vasodilation
tPBM can improve cerebral blood flow and lymphatic flow by acting on nitric oxide (NO) in two different ways. First, a photodissociation of nitric oxide previously bound to the complex occurs during the photon absorption in Cytochrome C Oxydase. Secondly, tPBM can stimulate nitric oxide synthase, the enzyme necessary for NO production in tissues.
Nitric oxide has vasodilatory effects. It thus leads to increased cerebral blood flow and cerebral lymphatic flow. This is crucial for supplying brain cells with nutrients and oxygen, removing waste products, and many other processes. Increased NO activity also leads to angiogenesis; the formation of new blood vessels.
A mouse model suggested that the effect size can be as big as 30%.
3) Improved cerebral oxygenation
Consistent with several animal studies, two human studies showed that transcranial laser stimulation can improve cerebral oxygenation in human adults. While total hemoglobin concentration only increased slightly to a non-significant portion, the oxygenated hemoglobin concentration increased and the deoxygenated hemoglobin concentration decreased, both significantly, leading to a significant increase in the share of oxygenated hemoglobin.
4) Decreased neuroinflammation
Neuroinflammation can contribute to the onset and progression of different neurological and psychological disorders, especially neurodegenerative diseases and depression, as they lead to neuronal loss and other detrimental effects and, thus, a decline in cognitive function.
tPBM can help decrease neuroinflammation in different ways. The mechanism can be simple – namely an improved removal of sources of inflammation such as metabolic waste products, neurotoxins and pathogens.
Through secondary effects, tPBM can also suppress the expression of proinflammatory cytokines.
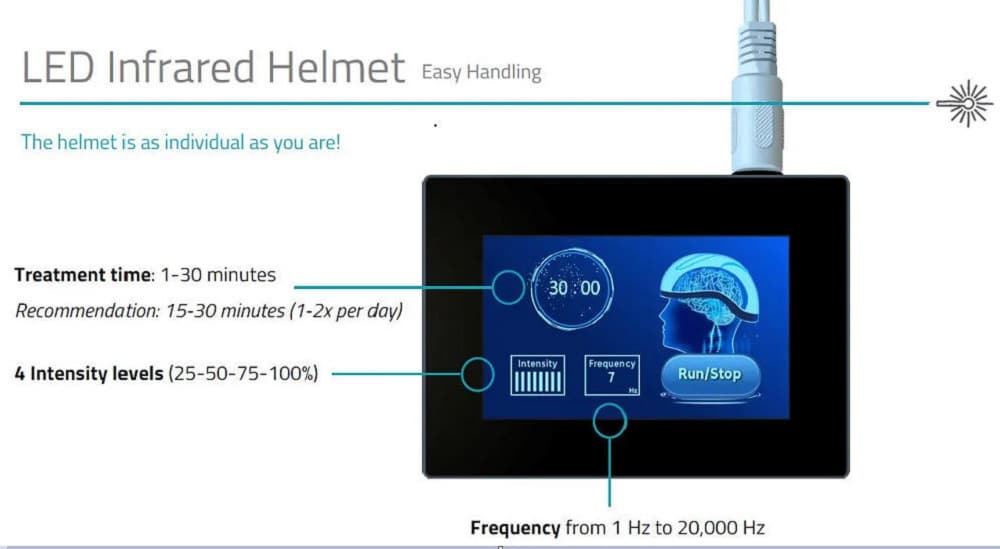
A rodent model suggested that tPBM may also work via modulation of the brain´s immune system. Microglia are an essential part of it and exist in two main phenotypes. “Microglia 1” are responsible for producing proinflammatory cytokines and interrupting the blood-brain barrier and are, in general, associated with neuronal damage. “Microglia 2” produce anti-inflammatory cytokines, enhance neurotrophic factor release, and have neuroprotective effects. The rodent model showed that tPBM could shift the balance of the prevalent microglia phenotype from M1 to M2.
5) Antioxidant effects
Long-term oxidative stress can contribute to neuronal loss, the disruption of neurocircuitry, a weakening of hippocampal, amygdalar, and cortical connections, and it can consequently lead to cognitive decline.
Due to the photon absorption in CCO, a brief and local burst of Reactive Oxygen Species occurs. This leads to the activation of antioxidant pathways in the body which reduces oxidative stress in the long run, despite ROS’s temporary and local increase.
6) Improved neurogenesis and synaptogenesis
Impaired neurogenesis is part of the neurobiology of different brain-related disorders and its correlation with cognitive impairment has been shown in different studies. It seems to play a particularly important role in neurodegenerative diseases such as Alzheimer´s and dementia, but also in Autism.
tPBM can improve the processes that underlie neurogenesis and synaptogenesis. The upregulation of the “Brain-Derived Neurotrophic Factor”—a signaling peptide involved in the maintenance and genesis of neurons and synapses—is the best-understood mechanism behind this effect.
7) Activation of signaling pathways and transcription factors that cause long-lasting changes in protein expression
tPBM activates multiple pathways through which signaling cascades occur, resulting in a long-term protein expression change.
The three best-understood pathways through which tPBM acts are:
a) The “calcium ions (CA²+) path” which plays a crucial role in multiple cellular processes such as cell proliferation, differentiation, adhesion, migration and survival.
b) The cyclic adenosine monophosphate (cAMP) which participates in neural plasticity, cognitive function and mitochondrial biogenesis.
c) NF-kB pathway which is activated by the brief and local burst of reactive oxygen species. The pathway is involved in regulating many critical cellular behaviors, particularly inflammatory responses, cellular growth and apoptosis [14].
Would you like to learn more? We can provide you with a literature list and other information material. Contact us through our MedicalExpo profile or send an email to martin.junggebauer@gmail.com.