heat-it is a medically confirmed, chemical-free principle of local hyperthermia that relieves the itch and pain of affected skin area, heated to 124 °F.
On display for the North American medical technology community at WHX in Miami last month, heat it® stood out among wellness-oriented digital health devices for its compact, user-driven innovation. This smartphone-powered insect bite relief solution is designed around a simple, well-studied principle: concentrated local hyperthermia. With a sleek plug-in form factor and app-based interface, the device addresses one of summer’s most common medical complaints—itching and inflammation from insect bites—without relying on chemicals or pharmaceuticals.
The growing attention surrounding heat it® is not only a result of its commercial success and design accolades but also of its clinical validation. Among other awards, it recently won the 2024 Outdoor Retailer Innovation Award, was featured on The Today Show as a top gadget at CES, and earned the top spot in Stiftung Warentest’s independent testing of insect bite relief products. These recognitions collectively underline its medical promise, particularly for general practitioners and pediatricians seeking non-pharmacologic interventions for pruritic skin reactions.
Clinical Validation: Hyperthermia as First-Line Relief
The fundamental principle behind heat it® is the use of controlled heat to alleviate the symptoms of insect bites, such as pruritus and localized pain. A pivotal study conducted by Charité – Universitätsmedizin Berlin, one of Europe’s most esteemed teaching hospitals, analyzed over 12,000 insect bites from 1,750 participants. The study found statistically significant reductions in itch intensity, with a 63% decrease within two minutes post-treatment and a 78% reduction after ten minutes. Published in Acta Dermato-Venereologica, the peer-reviewed results support the use of targeted hyperthermia as a rapid-onset, drug-free intervention.


The researchers employed a randomized, controlled trial to assess efficacy and safety. Participants used the heat it® device immediately following a bite or sting from common arthropods such as mosquitoes, horseflies, bees, and wasps. Results confirmed the safety of brief hyperthermic exposure at approximately 124°F (51°C), with no significant adverse events.
The pain-relief mechanism is hypothesized to involve nociceptive desensitization and potential histamine degradation at the dermal-epidermal junction—both topics of ongoing research in dermatology and immunology.
Consumer and Clinical Market Acceptance
In April 2025, Stiftung Warentest—Germany’s leading consumer watchdog—ranked heat it® as the top insect bite relief device out of 14 tested products. It was the only device to receive a flawless rating, scoring high for usability, design, and effectiveness. Stiftung Warentest’s influence in German-speaking markets is considerable, often shaping both consumer behavior and physician recommendations for OTC health tools. For primary care physicians, this provides external validation that can support patient adoption, especially among families seeking non-steroidal, non-antihistamine options for bite-related symptoms.
The engineering team behind heat it®, Kamedi GmbH, designed the device with medical versatility in mind. The product plugs into a smartphone’s USB-C or Lightning port and is controlled via a dedicated app. This allows for precise thermal control and personalization, with 12 adjustable treatment settings suitable even for pediatric and sensitive skin cases. A single phone charge allows up to 1,000 uses, eliminating the need for batteries and consumables—an important consideration for both clinicians and environmentally-conscious patients.

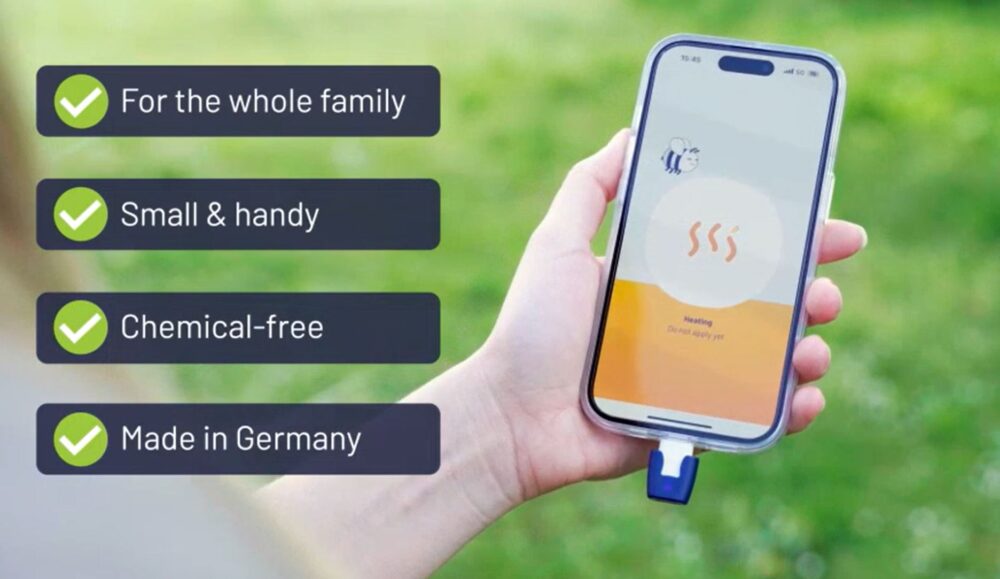
Usability and Practical Applications in Primary Care
For general practitioners, especially those in family medicine and pediatrics, heat it® offers a viable recommendation for patients seeking proactive or reactive bite management during outdoor activity seasons. The device’s compact size (keychain-friendly) and intuitive interface make it particularly attractive for families with children, travelers, and patients with dermatographic sensitivity to topical creams. Unlike antihistamine or corticosteroid-based treatments, heat it® offers immediate relief without systemic exposure or contraindications for concurrent medication use.
Additionally, the unit’s portability and digital integration open avenues for use in low-resource or mobile care environments. It is particularly well-suited for travel clinics and telemedicine practices that provide outdoor and adventure health consultations. With growing interest in digital therapeutics and patient-empowered care, heat it® fits into broader trends of micro-devices supporting self-administered, app-guided medical interventions.
Looking forward, this innovation could become a valuable tool in public health efforts to reduce secondary skin infections and scratching-related complications from insect bites. It also addresses a critical need for safe, immediate interventions during tick or vector encounters in remote environments—a key area of concern for wilderness medicine.
heat it® exemplifies how evidence-based design, combined with user-centric engineering, can bring clinical-grade utility into the hands of everyday users. For primary care physicians, it offers a low-risk, high-compliance adjunct to conventional bite management strategies, supported by compelling research and international recognition. As summer progresses and the outdoors call, heat it® represents more than just a gadget—it’s a medically validated extension of modern first aid.