Surgically implanted BCIs are advancing into clinical use, while Subsense’s nanoparticle-based, non-surgical approach is set to become a next-generation platform that could make brain-computer interfaces safer, more accessible, and broadly applicable across medicine and beyond.
When NPR recently highlighted the growing momentum of surgically implanted brain-computer interfaces (BCIs), the story underscored a remarkable turning point: after decades of research, these devices are entering clinical practice. Companies like Neuralink, Synchron, and Precision Neuroscience are pushing forward with implants that allow patients with paralysis or ALS to control cursors, communicate, and even experience sensory feedback.
But while these headlines capture the imagination, surgery itself imposes limits. Invasive procedures carry risks, regulatory hurdles, and barriers to adoption that make them suitable only for the most severe conditions. For many patients—and certainly for broader applications—less invasive options are needed.
The Limits of Surgery
Surgically implanted BCIs have proven their potential—patients have spoken, moved robotic arms, and even regained partial sensory feedback. Yet the road to widespread adoption is narrow.
First, there is surgical risk. Even minimally invasive implants require drilling into the skull or threading electrodes into the brain. For individuals with severe paralysis, the potential reward outweighs the risk. But for broader populations—including patients with Parkinson’s, Alzheimer’s, or epilepsy—the calculus becomes far less clear.
Second, regulatory and cost barriers remain steep. Clinical trials for implanted neurodevices can run into hundreds of millions of dollars, with long timelines for FDA clearance. Hospitals must also weigh the infrastructure costs of surgical implantation and long-term monitoring.
Finally, there is patient hesitancy. Surveys consistently show reluctance toward elective brain surgery, even when potential benefits are significant. The stigma of “a chip in the brain” may further slow adoption beyond the most urgent medical cases.
These factors create a ceiling: surgical BCIs are revolutionary but unlikely to reach mass clinical use. That reality opens space for new categories of brain interfaces that are safer, cheaper, and more acceptable to patients, such as non-surgical, nanoparticle-based systems.

The BCI Market
The commercial race in BCI is accelerating. Neuralink, Precision Neuroscience, Blackrock Neurotech, Paradromics, and Synchron are all vying to bring first-generation systems to market. Early clinical targets include restoring communication in ALS, enabling motor control for paralysis, and treating neurological disorders like Parkinson’s and epilepsy.
Neuralink is developing a fully implanted, high-channel interface with flexible probes inserted robotically into the cortex. Recent human trials include participants controlling devices with thought alone; one patient with ALS regained communication ability and even typed messages using a cursor controlled wirelessly by the implant’s electrodes (New York Post, Indiatimes, The Times of India).
Precision Neuroscience, founded by ex-Neuralink leaders, is targeting a minimally invasive cousin to traditional implants. Its ultra-thin, 1,024-electrode Layer 7 Cortical Interface conforms to the brain’s surface via a sub-millimeter cranial slit, avoiding penetration. The device has FDA clearance for up to 30-day use, having been used in dozens of patients during other neurosurgical procedures (Business Insider, Fierce Biotech).
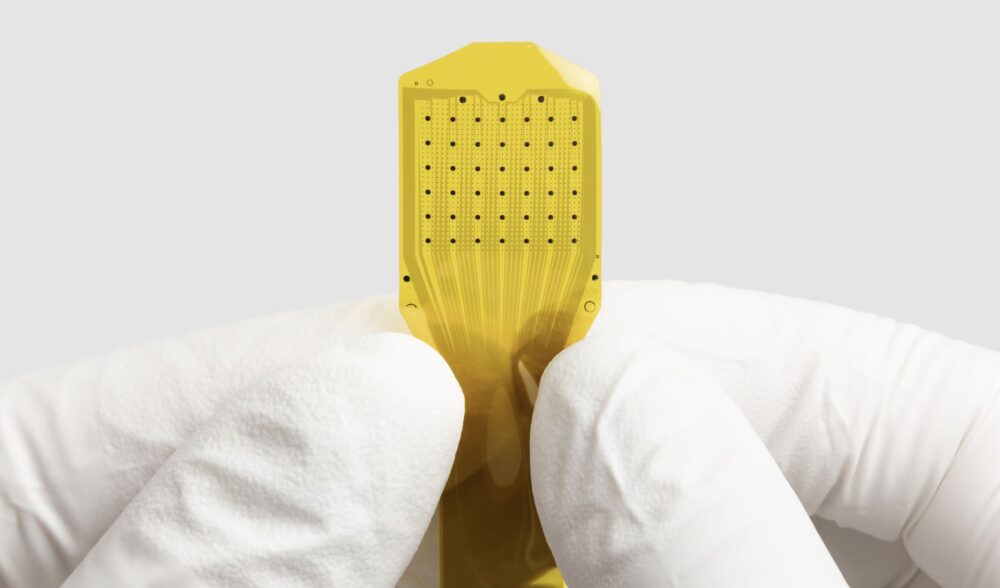
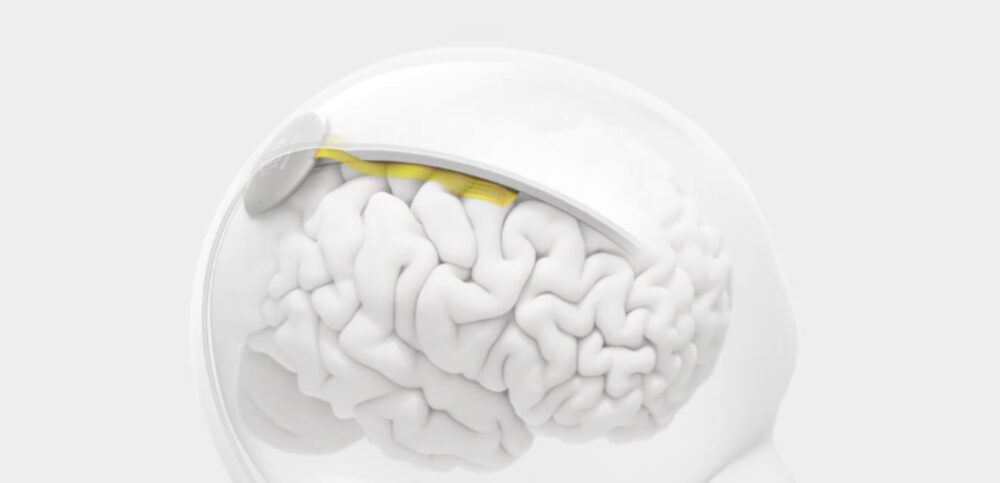
Blackrock Neurotech continues to deliver proven implantable systems such as the MoveAgain BCI, Neuralace arrays, and research tools like the Axon-R (non-invasive EEG), enabling movement, sensation, and speech restoration in both clinical and research settings (blackrockneurotech.com, BioSpace, allhealthtech.com).
Paradromics offers the Connexus BCI, which records at single-neuron resolution and decodes signals via AI for applications like real-time speech and motor control. The device has been safely tested in its first human implant and carries Breakthrough Device Designation and FDA engagement (paradromics.com, Bioworld).
Synchron takes an endovascular path: its Stentrode is implanted via the jugular vein—no open-brain surgery needed—and accesses motor cortex signals through the vasculature. Clinical studies show safe deployment, stable year-long signals, and meaningful digital control by patients (Synchron, Endovascular Today, WIRED, Fierce Biotech).
Meanwhile, Subsense positions itself distinctively: deploying magnetic-steered nanoparticles intranasally or across the blood-brain barrier for both recording and stimulation. This non-surgical, platform-based model promises flexibility and broader accessibility for future brain health treatments.
READ our interview with the team at Subsense to learn more.
Image: Courtesy of Subsense
BCI Case Studies Show Progress
Case studies already illustrate how transformative BCIs can be. NPR reported on patients regaining communication through thought-to-text systems, restoring movement via robotic arms, and even recovering a sense of touch through sensory feedback loops. These advances highlight both the promise and limitations of current implant-based devices.
For example, Neuralink’s early participant, paralyzed from the shoulders down, described the experience of moving a cursor with his thoughts as “freakin’ wild”. Yet technical setbacks—such as electrode retraction—show how fragile implanted hardware can be. Similarly, BrainGate’s long history of research demonstrates the potential of speech decoding, but it remains tethered to specialized clinical settings.
The challenges ahead are formidable. Technical risks include maintaining stable long-term signal quality, minimizing immune responses, and managing massive neural data streams. Ethical questions—around consent, data privacy, and equity of access—remain unresolved.
Subsense’s nanoparticle approach does not erase these issues, but it reframes them. Instead of questions of surgical safety, the focus shifts to nanoparticle biocompatibility, control of degradation, and ensuring precise targeting within the brain. If early preclinical studies succeed, this could reduce major barriers to adoption and open entirely new therapeutic frontiers.
Beyond Science Fiction
Surgically implanted BCIs are no longer science fiction. They are entering clinical reality for patients with profound disability. That is the present.
Subsense points toward a different future. By engineering nanoparticles to cross into the brain and interact with neurons without surgery, the company envisions BCIs that are safer, scalable, and flexible enough to support both clinical and non-clinical applications.
For medical professionals, this shift carries weighty implications. If implants represent a niche solution for the most severe cases, nanoparticle BCIs could redefine brain health on a broader scale, making neurotechnology not just extraordinary, but ordinary.