Forum Labo Paris 2025, organized by CIFL, showcases the evolving laboratory industry. As a proud media partner of the forum, we spoke with Loic Thomas, President of CIFL, during the event. Matthieu Boyer, General Manager of SARSTEDT, joined us to discuss the consolidation of medical labs, advancements in automation and AI, and the growing focus on predictive medicine.
25 – 27 March 2025—As Forum Labo Paris 2025 reaches new heights, it stands as the premier gathering for the laboratory industry in France, bringing together professionals from across the fields of research, analysis, and diagnostics. Organized by CIFL (Comité Interprofessionnel des Fournisseurs du Laboratoire), the Forum continues its tradition of fostering innovation and collaboration within the scientific community.
With over 300 exhibitors and more than 10,000 visitors expected this year, Forum Labo has evolved into a major event that reflects the latest trends and technological advances shaping the future of laboratories. According to Loïc Thomas, President of CIFL, the event has “grown exponentially,” offering a critical platform for companies to showcase cutting-edge products and solutions across various sectors.
Founded in 1960, CIFL represents laboratory suppliers across France and has been the driving force behind Forum Labo for decades. The organization serves as a unifying entity for its members, working actively to promote scientific professions and helping businesses stay ahead in a rapidly changing market.
Matthieu Boyer, General Manager of SARSTEDT, highlights that CIFL’s role extends far beyond the event, offering invaluable support through regulatory updates, market studies, and educational initiatives.
“CIFL is more than just an association,” Boyer stated. “It’s a hub for collaboration and innovation, helping small and large companies alike navigate the complexities of the laboratory market.”


Forum Labo: A Legacy of Innovation and Growth
Forum Labo has been at the heart of the laboratory industry since its inception in the 1970s under the leadership of CIFL. Originally known as the Laboratory Forum, the event rebranded to Forum Labo in 1994, and since then, it has become the go-to exhibition for companies specializing in laboratory instruments, consumables, and services. As Loïc Thomas explained:
“This year marks the 18th edition, and we alternate between Paris and Lyon to reach a broader audience.”
The event not only serves as a platform for showcasing the latest products but also fosters relationships between suppliers and professionals in fields as diverse as medical diagnostics, pharmaceuticals, food, and petrochemicals.
The growth of Forum Labo mirrors the expanding scope of the laboratory industry itself. The exhibition covers everything from furniture and consumables to cutting-edge technologies like next-generation sequencing and AI-driven diagnostics.
“The laboratory market is vast, encompassing not only medical analysis but also quality control, R&D, and much more,” Thomas noted.
Forum Labo has evolved alongside these sectors, adapting to new technological challenges and regulatory demands while helping businesses stay informed of critical trends. The inclusion of conferences on topics such as artificial intelligence and sustainable laboratory practices ensures that Forum Labo remains at the forefront of industry discussions.
CIFL: Supporting a Diverse and Dynamic Industry
CIFL’s mission extends far beyond organizing Forum Labo. As a professional association representing over 220 members and 10,000 employees, CIFL plays a crucial role in defending the collective interests of the laboratory profession in France.
“Members range from small startups to global leaders, and CIFL provides them with the tools they need to succeed,” Thomas said.
These tools include up-to-date regulatory information, reliable market statistics, and specialized training programs. Through its commissions and inquiries, CIFL gathers essential data to help members adapt to new market dynamics and regulatory frameworks.

One of CIFL’s core functions is to foster collaboration between its members, particularly in navigating complex regulatory landscapes such as the REACH directive or the law governing hazardous materials.
“Smaller companies may not always have the resources to stay on top of every new regulation, but CIFL steps in to provide guidance and support,” Thomas explained.
By organizing frequent commissions and networking events, CIFL ensures that its members—no matter their size—are equipped with the knowledge and tools to stay compliant and competitive in a rapidly evolving market. In doing so, CIFL not only protects the interests of the laboratory industry but also helps advance scientific progress across France.
Medical Updates in Laboratories: Insights from Matthieu Boyer
According to Boyer of SARSTEDT, the laboratory industry, particularly in the medical field, has undergone significant transformation in recent years. A key factor driving these changes is the increasing consolidation of medical analysis laboratories, which Boyer attributes to tighter accreditation requirements and financial pressures.
“The decrease in government reimbursements for certain analyses has forced laboratories to find new economic sources,” Boyer explained.
This shift has led to a geographic concentration of services, where smaller laboratories are closing, and larger, more centralized facilities are emerging. The concern, Boyer notes, is the impact on accessibility for patients in rural areas, where medical deserts are becoming more prevalent.
“If we close laboratories, patients may need to travel much farther for diagnoses,” he said, highlighting the growing need to balance financial viability with patient care.
In addition to structural changes, Boyer points out that advancements in technology are reshaping how medical laboratories operate.
“We are seeing more automation, AI integration, and the rise of molecular biology,” Boyer said.
These technologies are not only increasing the precision of medical analyses but are also shifting the focus from reactive to predictive medicine. With innovations like next-generation sequencing (NGS) and AI-driven diagnostics, laboratories are now able to anticipate medical conditions earlier and provide more tailored treatments. Boyer emphasized that these advancements are crucial for staying competitive in an increasingly data-driven healthcare landscape, noting that CIFL’s role in keeping its members informed of such technological trends is more important than ever.
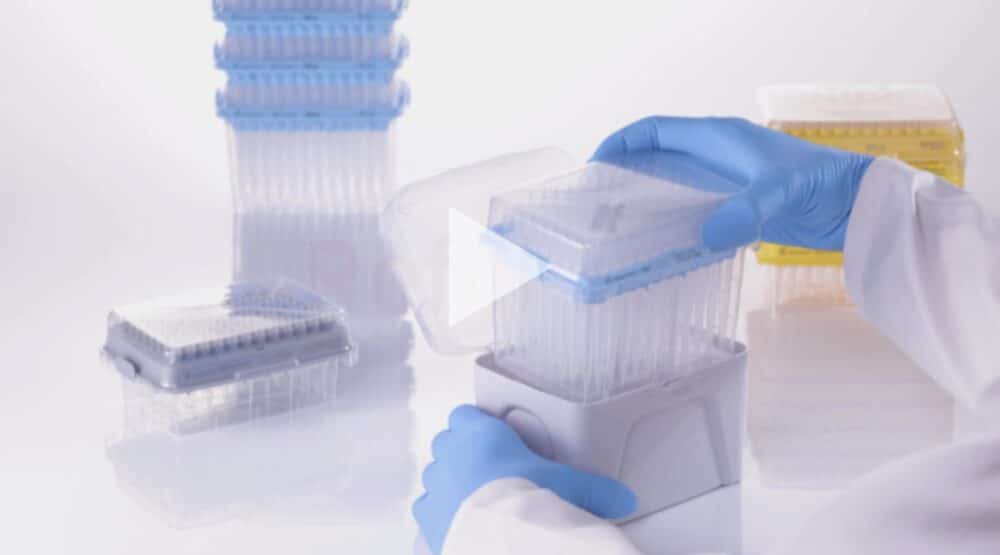
As for Sarstedt, the company presented its revolutionary boxing and re-filling concept for pipette tips. Laboratories around the world produce a lot of plastic waste due to the often necessary sterile disposable packaging. By combining the refill and recycling cycle the team at Sarstedt can conserve resources, save storage space and produce new laboratory products from recycled pipette tip boxes.
Forum Labo Paris 2025 and the efforts of CIFL continue to serve as pillars of the laboratory community, driving innovation, collaboration, and regulatory alignment in an industry poised for transformative growth.
READ our article about the growing interest in organoids: AI & Automated Solutions by Molecular Devices.