NurExone Biologic is leading research that could help restore lost neural function—offering new hope for patients with spinal cord or optic nerve injuries.
While the central nervous system (CNS) has limited capacity for repair, recent science shows that certain nerve cells can regenerate under the right conditions. However, natural regeneration is often too slow or insufficient to restore meaningful function after severe injury. As a result, damage to the brain, spinal cord, or optic nerves still typically leads to long-term or permanent disability.
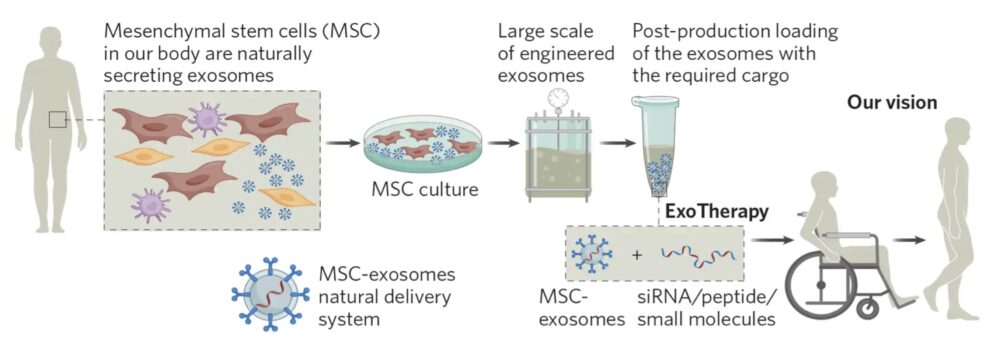
Israeli biopharmaceutical firm NurExone Biologic is aiming to change that. Its ExoTherapy platform harnesses the healing potential of exosomes—tiny, naturally occurring vesicles that act as cellular messengers, carrying proteins, RNA, and other molecular signals. Uniquely, these exosomes often travel from healthy to damaged tissues, making them powerful tools for targeted regeneration and repair.
Silencing Specific Genes to Initiate Nerve Cell Regeneration
The exosomes modulate the action of the immune system to reduce the inflammation the immune system causes so that regeneration can be promoted. Inflammation and regeneration are two mechanisms that contradict each other, Dr. Shaltiel explained.
“When you have a very strong action by the immune system, you do not have regeneration. It will not allow cells to grow. When you reduce inflammation, you have more room for regeneration,” Dr. Lior Shaltiel, chemical engineer and CEO of NurExone Biologic, told MedicalExpo e-Magazine.
These exosomes can be artificially “loaded” with various molecules, serving as a system that delivers drugs to a specific target area. In the case of spinal cord and optic nerve injuries, the exosomes are loaded with growth factors, DNA, peptides, and an active molecule that NurExone Biologic itself developed: the ExoPTEN, a specific siRNA (small interfering RNA). siRNAs are small double-stranded RNA molecules that work as a type of “signaler” to silence specific genes.
In the case of NurExone Biologic’s research, the protein silenced is the PTEN—a protein that has the power to stop cell growth. Therefore, when the loaded exosomes reach an inflamed or damaged area, they initiate an amazing process of nerve cell regeneration and recovery of function. “The exosomes work like guided missiles to inflammation. Inflammation is their target,” Dr. Shaltiel explains.
The nanodrug ExoPTEN has already received orphan drug status (a designation granted to medications developed for rare diseases) from the American Food and Drug Administration (FDA) and the European Medicines Agency (EMA). That gives the company substantial financial benefits and market protection.


The promising results
NurExone Biologic’s research has already shown impressive therapeutic efficacy in the rehabilitation of nerve cells. Rats whose spinal cords had been completely severed began walking again, and others whose optic nerves had been damaged regained sight. The company is moving forward towards human clinical trials, with the first test expected for 2026.
In addition, NurExone Biologic has recently announced a new therapeutic indication from its research focused on the peripheral nervous system, which shows success in preclinical results for facial nerve regeneration following a short, minimally invasive treatment.
The firm’s collaboration with Sheba Hospital in the field of ophthalmology has also been a source of great news.
“This collaboration started with a very warm connection we have with the well-known ophthalmologist Dr. Michael Belkin. He is the creator of the Berkin laser machine and is not only an advisor but also an investor in our company. Right from the beginning we wanted to take our research to ophthalmology.
We had very strong results in terms of function recovery, which was measured through the use of retinal graphene electrodes. The healthy eye and the damaged eye that was treated with the exosomes showed similar activity after only 18 days. Now we are working to get more and more data so that people understand that these results are reliable and can be repeated,” says Dr. Shaltiel.
Other possible uses
The PTEN protein has been closely studied for the last 30 years, mainly by oncologists. After all, cancer is, by definition, a cell proliferation problem: cancerous cells cannot stop proliferating. Loading exosomes with new molecules makes this technology potentially useful not only for oncology but also for orthopedics and dermatology, for example. An Israeli company called Nano24 even used exosomes to improve lung function during the pandemic, for example. Last, traumatic brain injury is another strong candidate to benefit from treatments such as the one provided by the ExoTherapy platform.
“The most meaningful challenge we face right now is the fact that exosomes are a new generation of medicine. They represent a form of cell therapy that does not involve actual cells. This represents a change in concept, and when the concept is altered and a new method is introduced, most of the time, if not all the time, there is often a lack of regulation in place.
We have this challenge of writing down the manuscripts of what is needed for the approval of the drug. But we are seeing more patents and publications coming out that are about exosomes. With favorable results, more and more companies will join,” Dr. Shaltiel believes.
Expansion
The Israeli company NurExone Biologic was established in 2022 as a spin-off of academic research conducted at the Technion and Tel Aviv University. Shortly after its establishment, NurExone Biologic made an unusual move for startups in general and young biotech companies in particular: it went public at the Toronto Stock Exchange (TSXV) and has since been traded there as a public company, raising over 17 million dollars.
Since then, NurExone Biologic has also been listed at the OTCQB Venture Market (OTCQB:NRXBF) and the Frankfurt Stock Exchange (FSE:J90). Plus, it is planning to go public in the United States, where it has just opened a subsidiary manufacturing facility that will soon start producing exosomes.
This activity will be a new revenue stream for the company and will, as a consequence, work as a protecting factor for its investors. The idea behind the establishment of the subsidiary is to sell the exosomes to other companies—including for cosmetic use—as countries like South Korea, the Philippines, Indonesia, Mexico, and Switzerland already allow the use of exosomes for cosmetic purposes.