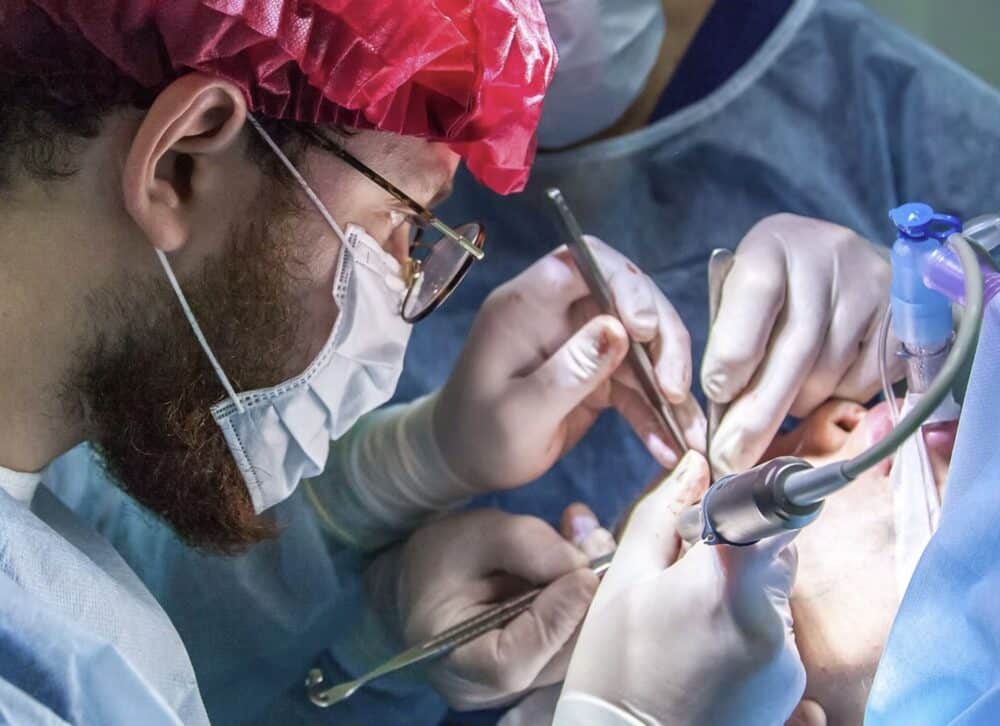
The Israeli health tech company CorNeat has developed a technology that mimics a biological collagen mesh with a vital function in the human body. The EverPatch+ Shield and the gPatch are the two most recent technologies.
Since its establishment in 2015, CorNeat Vision has been tackling the global shortage of corneal graft tissue with its synthetic solution, EverMatrix™. This innovative technology provides a scalable and biocompatible alternative to donor tissue, addressing the urgent need for corneal transplants. With approximately two million new cases of corneal blindness reported annually and only one available donor cornea for every 70 required, the need for a reliable, synthetic alternative is critical. CorNeat’s EverMatrix™ is designed to seamlessly integrate with human tissue, offering a permanent and effective solution for restoring vision while eliminating the risks associated with biological transplants.
“The CorNeat EverMatrix tissue-integrating technology has applications in almost every field of surgery and is poised to redefine the interface between implants (meaning synthetic materials) and surrounding living tissue,” co-founder, CEO & VP of Research & Development Almog Aley-Raz told MedicalExpo e-Magazine.
At the core of CorNeat’s success is EverMatrix™, a 100% synthetic, non-degradable, and porous nanofabric that mimics the extracellular matrix (ECM), a natural collagen mesh essential for cellular support and regeneration. This biomimetic technology enables the development of next-generation ocular implants, including the CorNeat KPro, a synthetic cornea, and the EverPatch+ Shield, a novel scleral reinforcement patch. Beyond ophthalmology, EverMatrix™ has also expanded into periodontal applications with the gPatch, a permanent solution for gingival tissue regeneration. With FDA clearance for the EverPatch and ongoing preclinical trials for the gPatch, CorNeat Vision continues to push the boundaries of synthetic tissue integration, redefining the future of surgical implants.
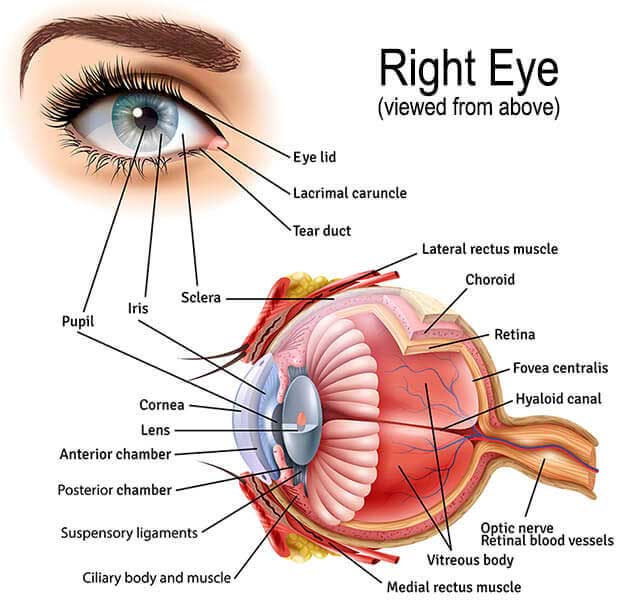
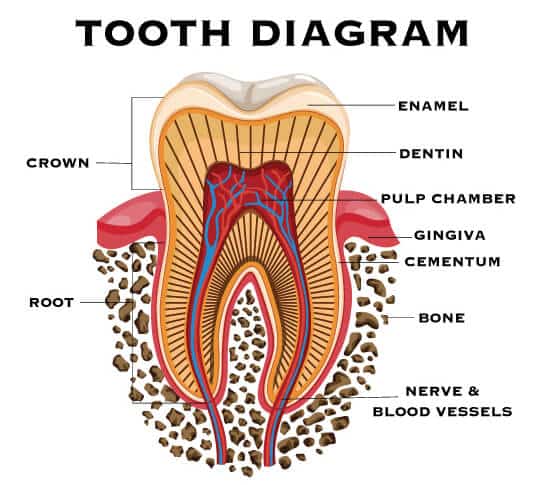
The First Inert, Synthetic, Non-degradable Tissue Substitute for Use in Ophthalmic Surgeries
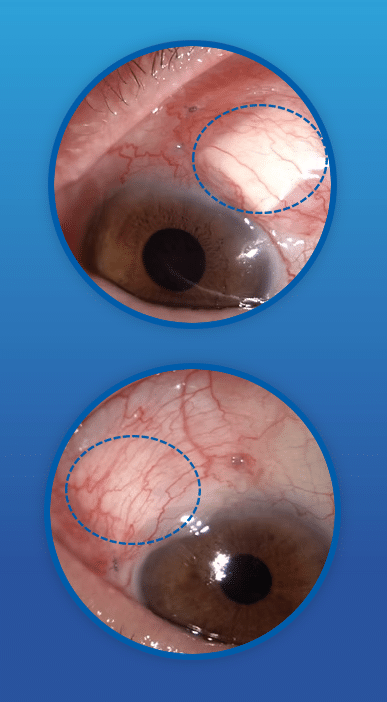
CorNeat’s EverPatch+ Shield is a new iteration of the EverPatch—the very first inert, synthetic, and non-degradable tissue substitute for use in ophthalmic surgeries. These characteristics make it so that the inflammatory response after the biomechanical integration is much less significant and temporary. The EverPatch+ Shield can also be used to cover uncomfortable implants, such as glaucoma tube shunts, or exposed sutures.
The EverPatch+ Shield is made of a non-woven polymer matrix, which integrates with surrounding tissue and reinforces the eye’s sclera, aiding the physical reconstruction of the ocular surface. The product has the potential to replace the use of donor and processed tissue, which eventually degrades and poses a risk of disease transmission.
Reconstructive surgeries of the eye are recommended after irreparable damage to the organ’s integrity. Such damage can result, for instance, from trauma, iatrogenic damage (that is, when a given medical treatment ends up causing tissue or organ damage) or an inflammatory connective tissue disease process. In these cases, patching the affected area with preserved and processed tissue is the only option.
Reduce Tension, Operating Time, and Costs
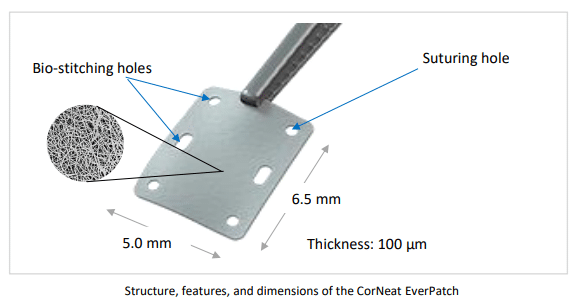
The EverPatch+ Shield is also extremely thin (100 microns). As stated by The Ophthalmologist last May:
“Where current tissue patches are thicker than 400-500 microns to allow for protection until native scar tissue forms to conceal the tube, the non-degradable EverPatch is much thinner, which reduces tension on the surgical wound and makes closing it easier for the surgeon.”
Demonstration video: https://www.corneat.com/everpatch-animation.
The product also cuts down on operating time and eye bank costs, as it doesn’t require the complicated handling and storage of biological materials. It also has a much longer shelf life than that of organic or degradable solutions.
The CorNeat EverPatch was granted FDA 510(k) clearance in June 2023, and the company is currently working closely with its regulatory advisors to obtain CE Marking for the product.
The First Permanent and Tissue-integrating Periodontal Surgical Matrix
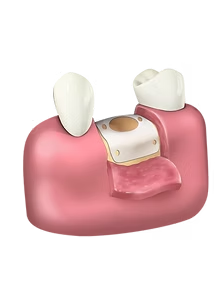
Another CorNeat novelty employing the EverMatrix™ is the gPatch, the first permanent and tissue-integrating periodontal surgical matrix. It can be used in gingival recession surgeries or Guided Bone Regeneration (GBR), a procedure where the alveolar ridge of the tooth is rebuilt through the increase of volume and dimensions of the gingival bone, in order to enable it to support a tooth implant.
Sponsored by the United States Navy, the gPatch combines the best features of non-degradable membranes (such as malleability and ease of handling and suturing, which leads to better bone regeneration control) and resorbable membranes and ECMs (like better biocompatibility and bio-integration in a single surgery, stimulating cellular growth, and maintaining nutrient flow).
Although gingival recession is a common problem in adults over the age of 40, it can occur in patients as young as 10. Also known as receding gums, it is characterized by the exposure of the roots of teeth due to the loss of gum tissue and/or retraction of the gingival margin from the crown of the teeth. Gingival recession is mostly caused by gum disease and brushing too hard. Receding gums may remain unnoticed until unpleasant symptoms such as tooth mobility and over-sensitive teeth appear.
Permanent Infrastructure for Gingival Tissue Regeneration
Current solutions for gingival recession rely on the use of grafts and collagen matrices, which are stitched over the exposed area and then covered by a flap of soft gingival tissue. However, our bodies absorb these materials over time, leading to poor long-term results. The gPatch, on the other hand, provides a permanent infrastructure for gingival tissue regeneration.
“The CorNeat gPatch is the only product outside of ophthalmology that we are developing at the moment. It is the only ‘scaffold’ that remains in the tissue for life and, once bio-integrated, provides long-lasting mechanical support to surrounding tissue. The gPatch will disrupt the gingival soft tissue repair market and revolutionize current treatment,” Gilad Litvin, co-founder and Chief Medical Officer (CMO) of CorNeat, told MedicalExpo e-Magazine.
Just like the EverPatch+ Shield, the gPatch is fully synthetic. Therefore, it cannot carry or transmit diseases and offers a major advantage from a logistical perspective, as that allows for a long shelf life and fewer regulatory hurdles related to transportation. Plus, it does not require removal after surgery. Easily cut, fixated, and sutured to the target site, the EverPatch+ Shield is tear-resistant, stretchable, and also a space maintainer.
The gPatch was proven safe and effective in subcutaneous and sub-gingival implantations. Throughout 2024, the product went through biocompatibility tests and additional preclinical in vivo trials, which included a (large) animal GLP implantation study. The last tests are still ongoing.