The Aeson innovative artificial heart from French medtech firm Carmat could be a game changer for those with advanced-stage heart failure. With this device, Carmat aims to meet a major public health challenge related to cardiovascular diseases, namely heart failure, which is the leading cause of death in the Western world. More specifically, the company wants to provide a lasting solution for the treatment of terminal heart failure, a disease for which there are very few effective options today, the main one being heart transplants.
A Growing Challenge
The human heart beats around 100,000 times a day, each cycle of contraction and relaxation circulating blood, oxygen and nutrients throughout the body. Yet when the heart can no longer carry out its essential function as a pump, vital organs such as the brain, liver and kidneys start to shut down.
The main symptoms of heart failure (HF) include fatigue, shortness of breath and fluid retention, while normal activities such as climbing stairs, engaging in domestic chores or even putting on clothes become increasingly difficult.
With coronary artery disease as the main cause, heart failure has become a global pandemic affecting at least 26 million people across the globe. And the pandemic is worsening. In the United States, for example, 5.7 million people currently suffer from HF—this figure is expected to reach more than 8 million by 2030.
Despite significant advances in therapies and prevention, those suffering from HF typically experience high mortality and morbidity and a poor quality of life.
Supply Versus Demand
Heart transplantation has been the optimum treatment for HF for many years. However, this is constrained by a scarcity of donors, which limits the number of transplants to about 5,500 lucky heart recipients every year.
This gap between supply and demand, which means many HF sufferers have to wait years for a donor, has driven the development of mechanical circulatory support (MCS) systems. These either help weakened hearts to pump blood (left ventricular assist devices or LVADs), or replace the human heart entirely (total artificial hearts or TAHs). They are typically used to bridge the time to heart transplantation or to permanently replace the heart if a transplant is impossible.

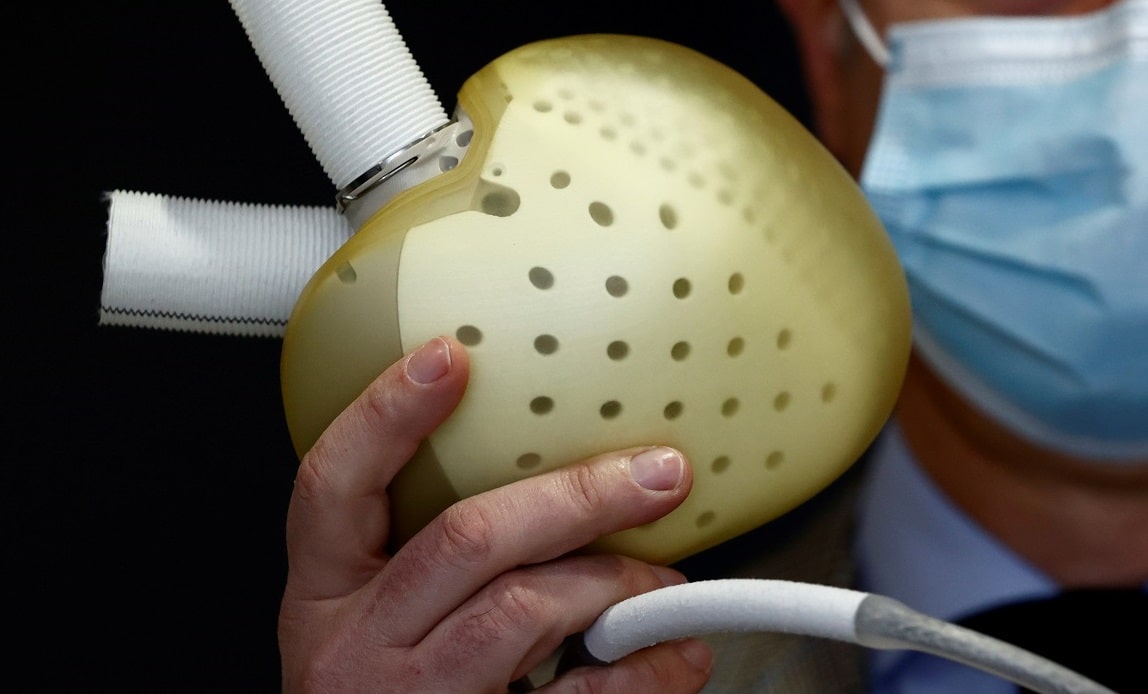
One of the most advanced total artificial hearts currently under development is the Aeson from Carmat, a French healthcare and medical technology company founded in 2008. The company’s ultimate ambition is to make Aeson the first non-organic alternative to a heart transplant and thus provide a therapeutic solution to people suffering from end-stage biventricular heart failure.
The sophisticated device, which Carmat claims is the first physiological artificial heart, has been approved by European authorities as a so-called “bridge-to-transplant” but not (yet) as a permanent implant.
Device Innovation
Compared to previous TAH devices, the Aeson boasts some unique features. Following a collaboration with technological experts from Airbus, its blood-contacting surface is made of bovine pericardium tissue, while it also employs bioprosthetic, bovine pericardial valves.
These enhance hemocompatibility, reducing the risk of blood clots or strokes in implant patients, and therefore the need to use anticoagulation agents such as heparin.
The Aeson also features an electrohydraulically driven, pulsatile flow mechanism that mimics native ventricular contraction. This works to reduce the shear force applied to blood elements (it is vital that right and left heart circulations are properly balanced in TAHs, otherwise pulmonary overcirculation may lead to edema or hemorrhage).

In addition, pump outputs are autoregulated on the basis of preload pressure changes measured inside the device. This reduces the need for patients to meet with doctors for post-implant adjustments.
According to Carmat, the Aeson provides the mobility and autonomy needed to lead a near-normal life. Associated external equipment weighs around 4 kg and includes a controller and two battery pockets providing an autonomy of approximately 4 hours. A medical team uses a hospital care console to operate the prosthesis during implantation and monitor how the device is functioning.
Development Timeline
In 2021, Carmat announced the first implantation of its Aeson total artificial heart in the United States within the framework of the Early Feasibility Study (EFS).
Aeson is currently also being assessed in a clinical study in France. The first Aeson implantation within the framework of this study was performed in December 2022 at Lille Regional University Hospital. In addition to the Lille hospital, five other medical centers take part in this study in the cities of Paris, Rennes, Strasbourg and Lyon.
This prospective study will involve a total of 52 patients eligible for a heart transplant in France and will allow Carmat to collect both additional data on the efficacy and safety of its artificial heart and medico-economic data to support its value proposition and the device’s reimbursement, notably in France. The study’s primary endpoint is survival for 180 days after implantation of the device without a disabling stroke, or a successful heart transplant within 180 days of implantation.
In accordance with the principles it has consistently applied, the company does not plan to communicate on the state of health of individual patients nor on the performance of each implantation. Carmat will continue to communicate on its progress when it reaches significant milestones and when it publishes its financial results.
While important questions need to be addressed, the device represents a step forward in the challenging TAH space and has the potential to save the lives of thousands of patients waiting for a heart transplant.











