At Brazil’s largest healthcare event, brain4care’s award-winning non-invasive brain monitoring technology stood out as a breakthrough in preventive, patient-centered care.
At CONAHP 2025—the National Congress of Private Hospitals—held on October 15 and 16 in São Paulo, one name resonated strongly among the more than 150 exhibitors shaping the future of healthcare: brain4care. Founded in 2014 by physicist Sérgio Mascarenhas and his research partners, the company has achieved what once seemed impossible—monitoring brain pressure and compliance non-invasively. This technology stems from Mascarenhas’s groundbreaking discovery that the human skull pulsates, challenging a long-standing medical assumption about cranial rigidity. The result is a sensor that gives physicians safer, faster access to crucial neurological data without the risks of traditional invasive methods.
At the event’s Future Health Circuit, brain4care’s solution stood out as a powerful example of how scientific insight and design can converge to improve outcomes. Recognized globally—with honors such as the iF Design Award 2024 and selection to the World Economic Forum’s Technology Pioneers community—the company embodies the innovation that defines Brazil’s growing presence in medtech. Its participation in CONAHP 2025 not only showcased its pioneering device but also underscored a broader shift: a future in which preventive care and real-time data make brain monitoring safer, smarter, and more accessible than ever.
The result is a sensor that gives physicians safer, faster access to crucial neurological data without the risks of traditional invasive methods.

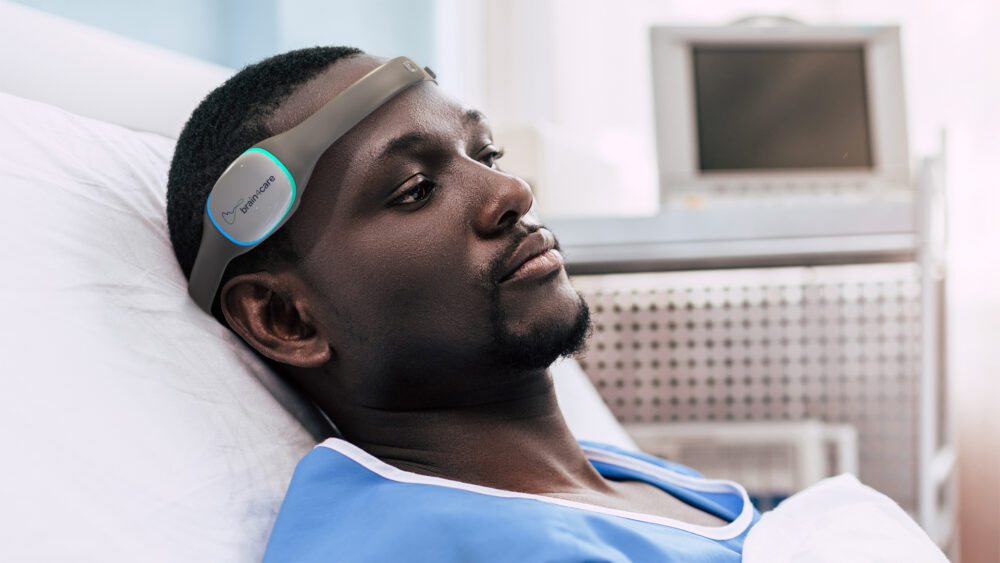
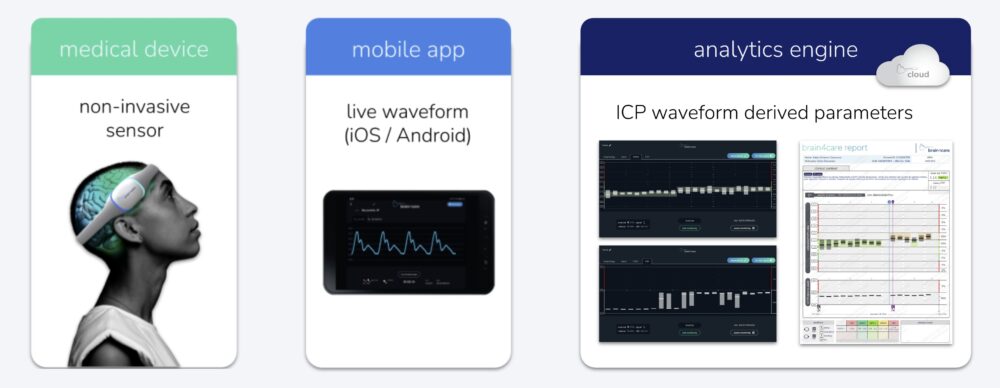
The brain4care: Non-invasive Sensor Monitoring and Cloud-based, AI-processed Data Analyzing
Among the products exhibited at CONAHP 2025 as a whole, the brain4care stood out as an exciting novelty. The creation is a non-invasive sensor that monitors patients’ complacency and intracranial pressure and is connected to a cloud-based platform that uses AI to process the data in real time, producing reports that help doctors make more well-informed decisions.
The main advantage of the brain4care sensor is that it allows doctors to take proactive action to reduce neurological damage and sequelae before the worst happens. Without this technology, obtaining data on intracranial pressure and compliance variations is only possible through invasive procedures, such as surgically inserting a catheter into the patient’s skull, which can be risky depending on the injury and state of the patient.
A Clinical Case: Swelling in the Brain and a Broken Bone in the Skull
That was precisely the situation of an 18-year-old Brazilian patient on whom the device was tested earlier this year. He had just been through a serious car accident and arrived at the hospital with severe craniocerebral trauma, the fourth lowest score (6) in the Glasgow Coma Scale (GCS), respiratory and hemodynamic compromise, and aspiration pneumonia. The tests revealed swelling in the brain, a broken bone in the skull, and a large collection of blood outside the brain, along with fractures in the collarbone and the second neck vertebra. The use of the catheter was not recommended due to clinical instability and thrombocytopenia (low number of platelets in the blood), which put him at a high risk of bleeding.
The medical team then decided to monitor the patient’s intracranial pressure through the brain4care between four and six times a day, which allowed for the adjustment of the medication regimen according to the evolution of intracranial dynamics and the monitoring of the risk of neurological damage.
Sixteen days later, the patient was out of sedation, discharged from the ICU, and scored 14 (the second-best score) in the GCS. In one more week, he was discharged from the hospital—with no rehospitalization or sequelae—after a clavicle surgery and cranial correction.
“Information on intracranial dynamics and compliance obtained non-invasively and at the bedside provides visibility into the diagnosis and treatment of patients at risk of, suspected of, or with confirmed intracranial hypertension or hypotension. This results in better clinical outcomes and reduces costs,” Arnaldo Betta, operations director at brain4care, told MedicalExpo e-Magazine.
Markets and Expansion: ICUs, Surgical Centers, and Elderly Care Clinics
With approval from Anvisa, Brazil’s Health Regulatory Agency (since 2017), and the Food and Drug Administration (FDA) in the United States (since 2021), brain4care has 44 global patents, 110 published scientific articles, and 90 clients—clinics and hospitals—in Brazil. Last June, the healthtech got its first client in the United States: the University of California San Diego.
“Our focus is on consolidating 15 American reference centers for the application of this technology in ICUs by the end of 2026. In parallel, we are conducting pilot validation projects for intraoperative use and are developing a strategic project aimed at the senior living segment. In Europe, we are advancing in the regulatory process, with research underway, and expect commercialization to start in the third quarter of 2026, focusing on ICUs, surgical centers, and elderly care clinics,” says Plinio Targa, CEO of brain4care.
Expansion is already taking place in Brazil and in the rest of Latin America, and a pilot project in the Middle East is about to start. The company currently has offices in São Carlos and São Paulo, both located in the Brazilian state of São Paulo, and Atlanta, in the United States, and has recently joined the World Economic Forum (WEF) Technology Pioneers community. The company was the only Brazilian firm to participate in the 16th Annual Meeting of the World Economic Forum’s New Champions 2025, which took place in July in China.