As the interest in brain health increases, we gather information on the therapies currently drawing attention in the medical industry.
As global populations age, preserving and enhancing brain health has become a central focus in medicine. Attention is converging not just on traditional pharmaceutical interventions, but on advanced technologies offering novel, non-invasive ways to maintain cognitive function, manage stress, and support neuroplasticity. Brain-computer interfaces (BCIs), AI–powered wearables, red light therapy, and integrative light-sound systems like BrainTap are among the tools receiving significant interest.
These modalities—complemented by emerging approaches such as craniosacral therapy, epigenetic nutrition, and vision-centered neuroplasticity training—span a spectrum from cutting-edge digital diagnostics to mind-body interventions. For clinicians, the challenge lies in discerning evidence-based utility amid a landscape rich with innovation and variable scientific validation.
Brain-Computer Interfaces (BCIs)
The current BCI landscape splits between invasive and non-invasive approaches. Invasive systems—such as Neuralink and Utah arrays—offer high-fidelity neural signals but carry surgical risks. Non-invasive methods (EEG, fNIRS, tDCS) are safer but less precise. Subsense introduces a paradigm shift with its nanoparticle-based, non-surgical BCI, delivering particles intranasally or via bloodstream and guiding them with magnetic fields to interface with neural tissue—combining recording and stimulation in a potentially accessible format.
Clinical targets for BCIs are expanding: from motor deficits in Parkinson’s and post-stroke rehabilitation, to communication restoration in ALS, seizure control in epilepsy, and mood modulation in depression. Long-term applications may include cognitive enhancement and support for neuroplasticity. However, challenges remain: the long-term biocompatibility of nanoparticles, tight regulatory classification between device and therapeutic, and protecting the privacy and security of neural data all demand rigorous evaluation.
READ our article in which we dive into the BCI market.

AI & Wearables: Clinical Evidence and Key Insights
AI-driven neuro-monitoring is transforming brain health through continuous, personalized data collection. Platforms like TruNeura enable clinics to scale brain health protocols—suggesting use of wearable sensors and AI analytics in routine care.
Artificial intelligence is rapidly becoming the backbone of personalized brain health, fueled by data streams from consumer and medical-grade wearables.
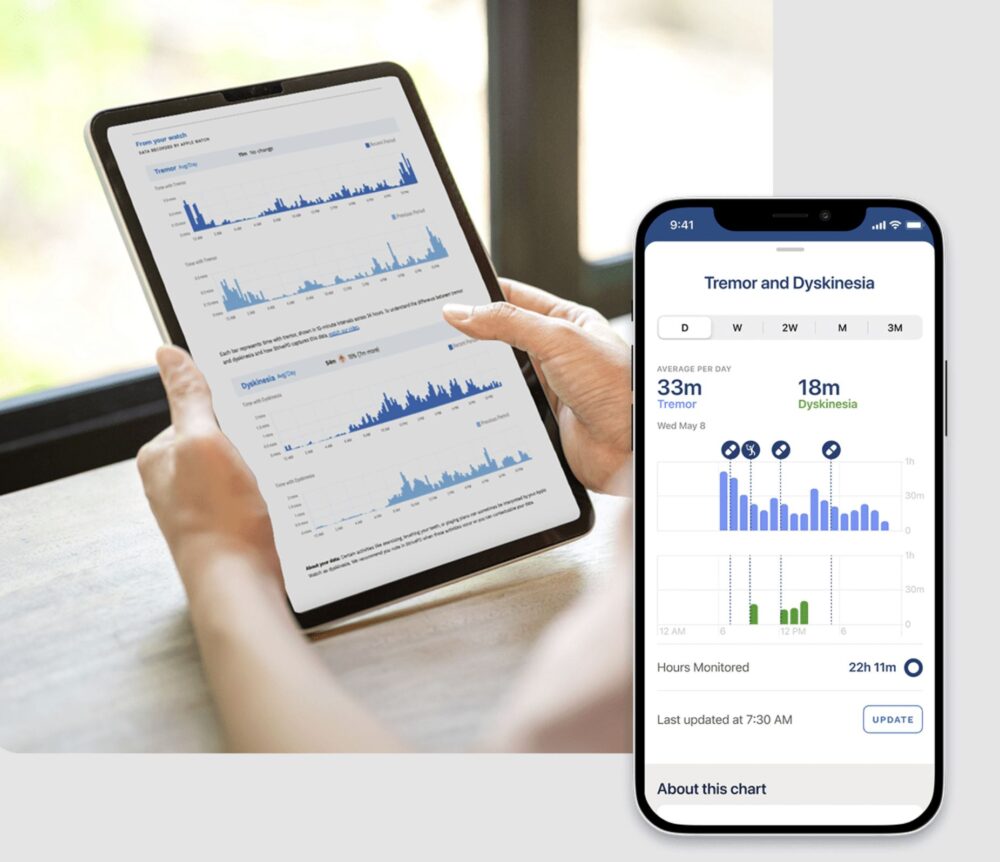
Rune Labs exemplifies this integration: their FDA-cleared StrivePD platform leverages Apple Watch movement data to track tremor and dyskinesia in Parkinson’s disease. Coupled with medication tracking, this provides clinicians with decision-support dashboards. Early deployment at Kaiser Permanente suggested reduced emergency visits, underscoring its potential clinical impact.
TruNeura takes a broader approach, offering clinics a platform to implement personalized brain health programs. It integrates wearable-derived metrics—heart rate variability (HRV), sleep cycles, stress indices—alongside coaching inputs to guide patient adherence.
NeuroStory.ai brings an AI-powered digital health layer, combining wearable data, cognitive assessments, and predictive modeling for brain health risks and resilience. While still early-stage, the model points toward scalable digital neurohealth programs.
Finally, practices like those of Sunjya Schweig, MD (California Center for Functional Medicine) demonstrate how continuous monitoring tools—CGMs, HRV trackers, EEG headbands—can be embedded in integrative care for patients at risk of cognitive decline.
Clinical evidence. Digital motor biomarkers for Parkinson’s are already FDA-cleared. Pilot data support HRV biofeedback and wearable-based stress retraining as effective in reducing autonomic dysfunction. While much remains in validation, clinicians should anticipate rapid adoption of digital biomarkers into precision brain health models.
Key takeaway. Wearables plus AI enable continuous, patient-specific insights into brain health. The next step will be validating which digital signals best predict disease progression and therapeutic response—work already underway.
Red Light Therapy (Photobiomodulation)
Photobiomodulation (PBM) applies red to near-infrared light (630–1100 nm) to cortical tissue, where photons are absorbed by mitochondrial cytochrome c oxidase. This stimulates ATP production, reduces oxidative stress, modulates nitric oxide signaling, and may improve cerebral blood flow and synaptic resilience.
CeraThrive is one company bringing PBM into a brain health context, positioning its devices around systemic and neurocognitive optimization. For clinicians, careful scrutiny of wavelength, irradiance, and dose is critical, as these parameters determine therapeutic impact.
Clinical trial data remain preliminary but promising. A 2024 randomized trial in post-stroke patients reported improvements in cognition and functional recovery following a 12-week PBM course. Similarly, a 2022 sham-controlled study on patients with persistent post-concussive symptoms showed reduced cognitive complaints and improved mood. Pilot data also suggest benefits in PTSD and mild cognitive impairment, though heterogeneity in trial design complicates interpretation.


Challenges. No standardized dosing protocols exist, and device quality varies widely across consumer and clinical markets. FDA approvals currently cover indications such as musculoskeletal pain and wound healing, but not broad neurological use.
Key takeaway. PBM holds potential as a low-risk, adjunctive therapy in neurorehabilitation and cognitive care, but widespread clinical adoption will require larger, standardized trials and clear dosing guidelines. Clinicians trialing PBM should carefully document treatment parameters and outcomes.
Light & Sound Therapy: BrainTap
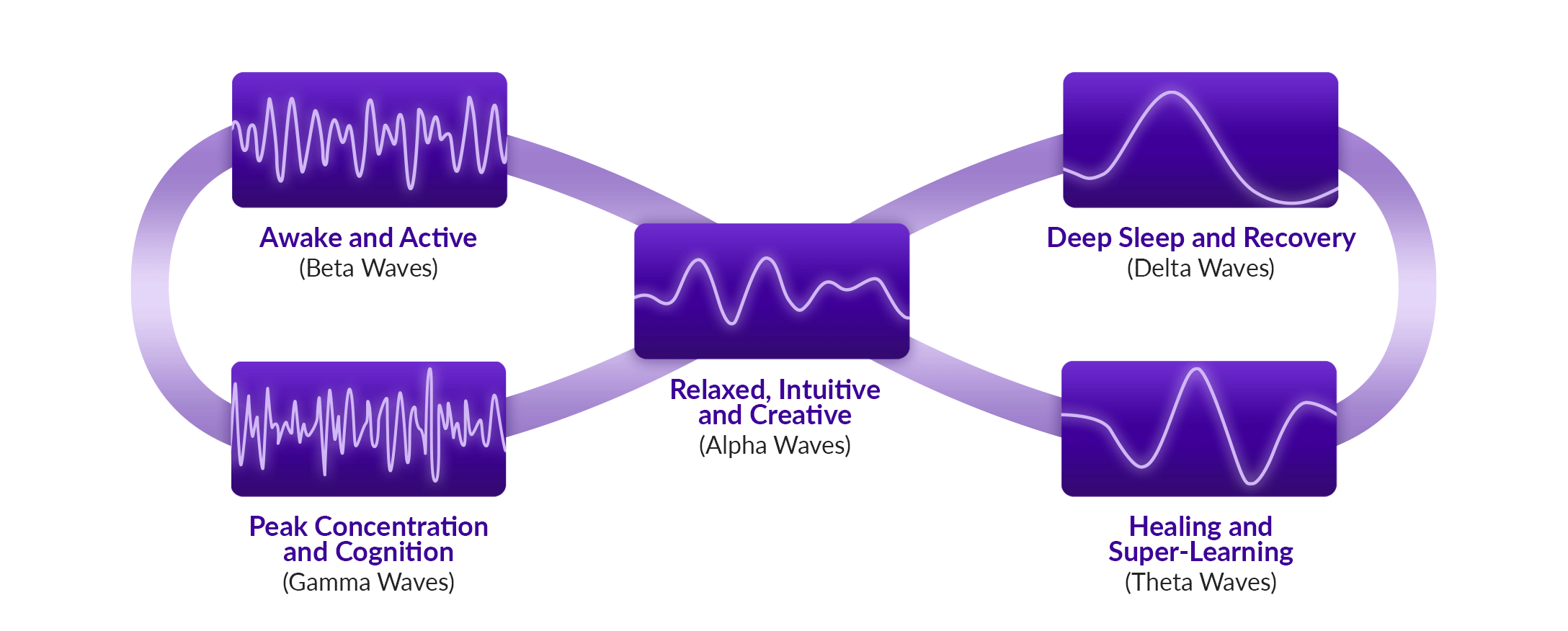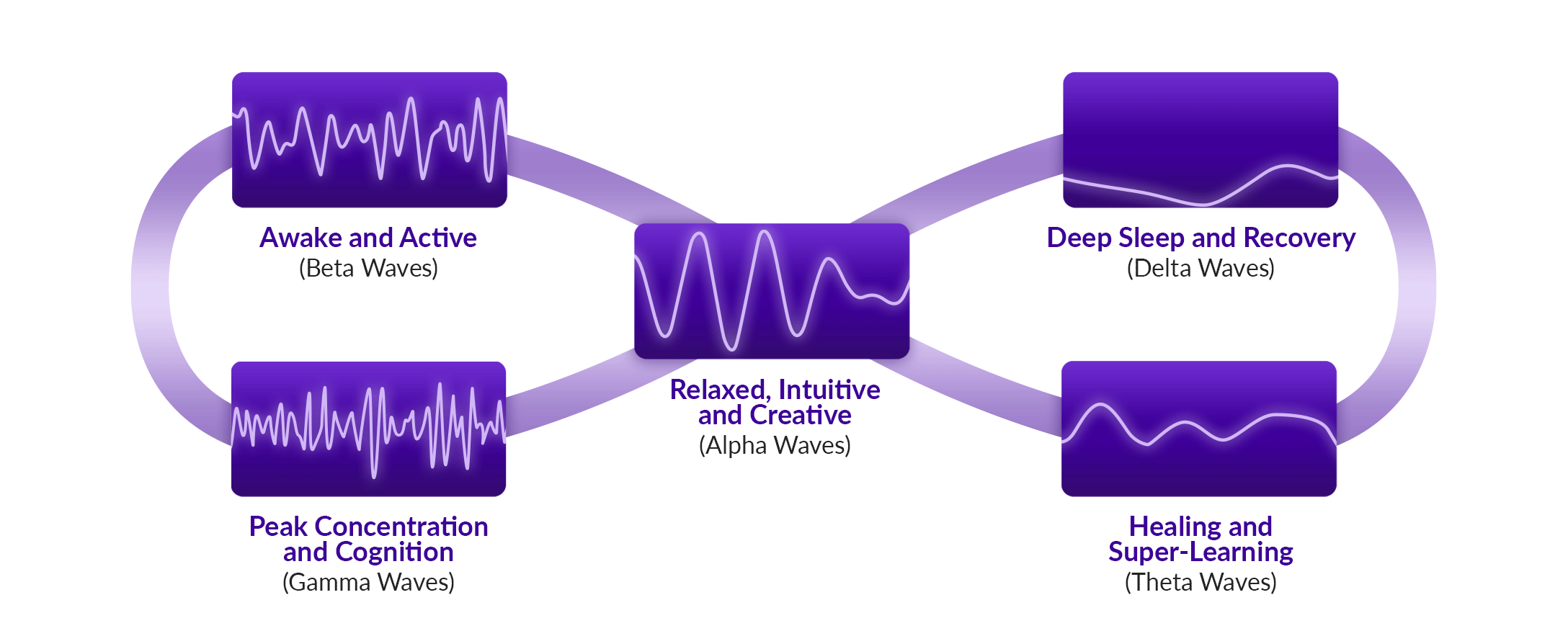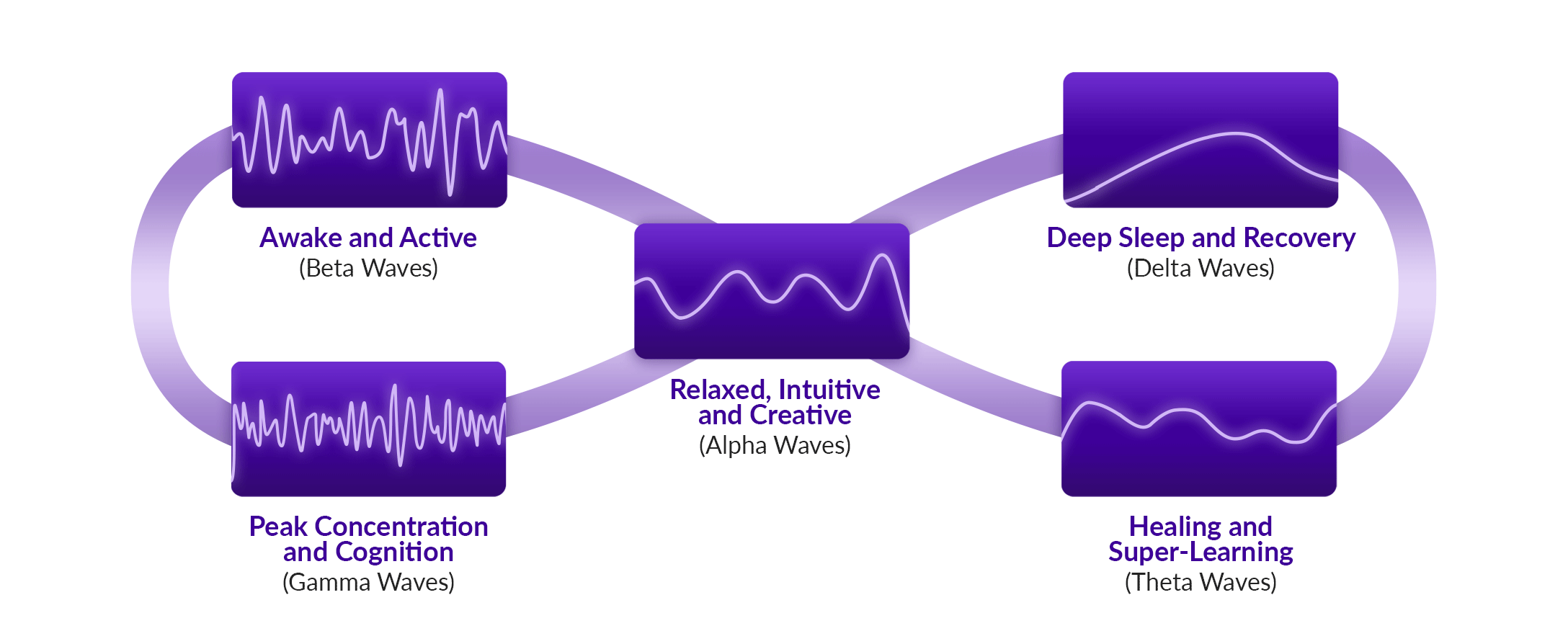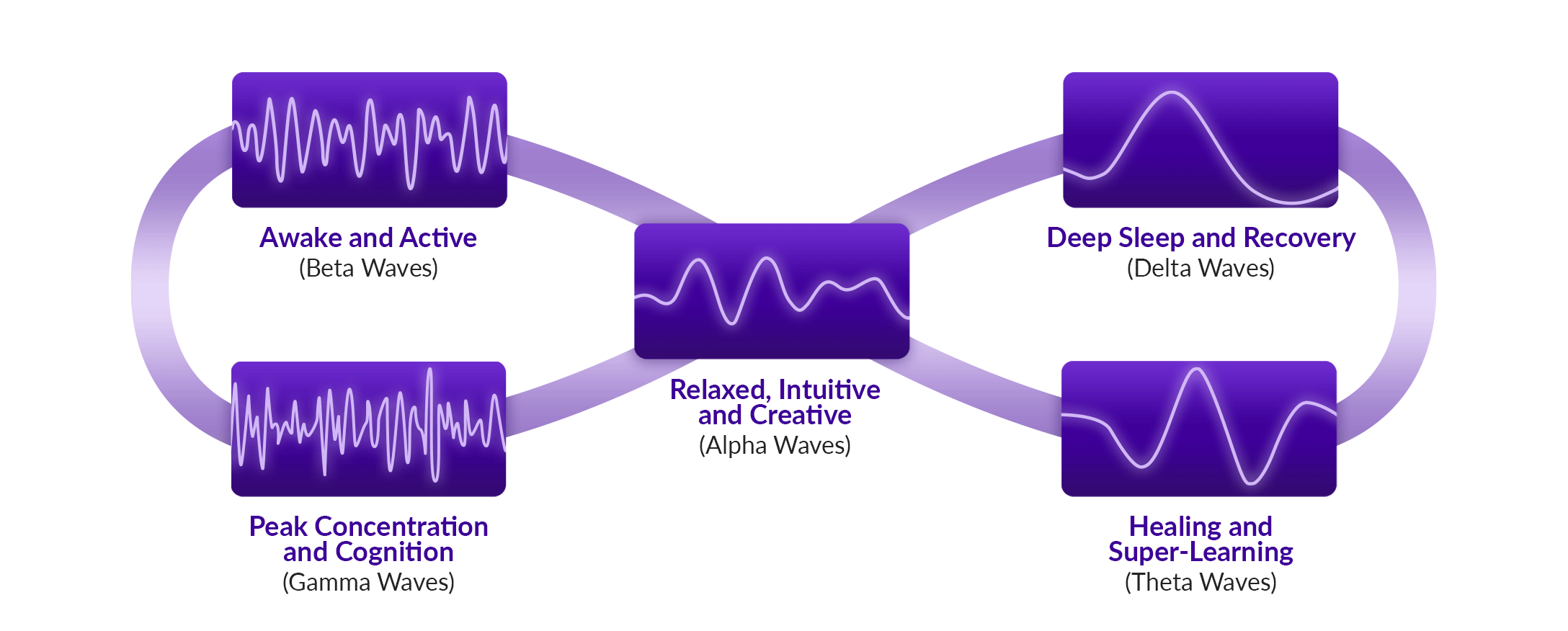
BrainTap combines light frequencies (via pulsed LEDs through a visor and earphones), binaural beats, isochronic tones, guided visualization, and holographic music to entrain brainwaves effectively—the process known as brainwave entrainment (BrainTap, info.braintap.com, Med House). These multisensory sessions aim to induce alpha, theta, and delta states to promote relaxation, focus, sleep, and stress reduction.
Multisensory entrainment therapies are gaining traction as tools for stress regulation and cognitive support. BrainTap, developed by Dr. Patrick Porter, integrates pulsed LED light (delivered through a visor) with binaural beats, isochronic tones, and guided visualization delivered via earphones. The system aims to entrain brainwave activity into alpha, theta, or delta states, supporting relaxation, improved sleep, and focused attention.
By synchronizing auditory and photic inputs, BrainTap seeks to influence thalamocortical rhythms and autonomic tone, similar to broader audio-visual entrainment (AVE) research.
Controlled studies of AVE and binaural beats demonstrate mixed but encouraging results, showing reductions in anxiety, improved mood, and measurable EEG entrainment. Importantly, recent trials of 40 Hz gamma stimulation—distinct from BrainTap but within the same multisensory domain—have shown slowed neurodegeneration in Alzheimer’s disease, with phase I/II data suggesting improvements in myelin integrity and functional outcomes.

Clinical considerations. BrainTap positions itself as a consumer wellness product, not a medical device. While small studies support AVE for stress and sleep regulation, robust randomized trials are still limited. Clinicians employing BrainTap or similar modalities should screen patients for photosensitive epilepsy and measure outcomes with validated tools such as HRV monitoring or anxiety scales.
Key takeaway. BrainTap represents an accessible, adjunctive modality with plausible neurophysiological mechanisms and preliminary evidence. Its role in medical practice remains supportive rather than curative, but ongoing research into light and sound entrainment may open therapeutic doors, especially in neurodegenerative disorders.
Other Relevant Therapies
- Craniosacral Therapy: A gentle manual modality targeting cerebrospinal fluid flow and cranial mechanics. Though data are limited, exploratory reports hint at benefits for dementia and trauma where gentle manual regulation may support brain-body balance.
- Epigenetic Nutrition: Pioneers like Kara Fitzgerald, ND (IFMCP) and Ari Whitten, MS advocate nutrition and lifestyle modifications to influence gene expression linked to aging and neurodegeneration. These interventions incorporate personalized diets, stress modulation, and environment to biochemically support brain resilience.
- Neuroplasticity Interventions (Vision Therapy, Mindfulness): Structured vision therapy, trauma-informed cognitive training, mindfulness, and physical exercise demonstrate strong evidence for enhancing neuroplasticity, recovery, and resilience. These are clinically validated adjuncts to technological interventions.
The emerging landscape of brain health reflects a convergence of biology, technology, and lifestyle, offering clinicians new possibilities that extend well beyond traditional pharmacology. Brain-computer interfaces demonstrate how direct neural access could restore lost functions, while AI-driven wearables promise continuous digital biomarkers that shift care from reactive to proactive. Adjunctive modalities such as red light therapy and multisensory entrainment platforms like BrainTap highlight how energy-based interventions may influence cellular and network-level dynamics, complementing conventional care.
At the same time, craniosacral therapy, epigenetic nutrition, and neuroplasticity-focused training remind us that the brain is responsive not only to devices but to environment, diet, and experience. Together, these diverse approaches illustrate a future in which brain health care is personalized, multimodal, and integrative—requiring clinicians to navigate both rigorous clinical evidence and rapidly evolving innovation.