ABIVAX’s novel oral therapy, obefazimod, shows promising results in treating refractory ulcerative colitis by targeting upstream inflammatory pathways.
In a webcast hosted on March 17, 2025, ABIVAX showcased their latest developments in ulcerative colitis (UC) treatment, presented by Dr. David T. Rubin, MD, a prominent gastroenterologist from the University of Chicago. The webcast centered on the ongoing phase 3 trial for obefazimod (ABX464), a novel oral therapy targeting the inflammatory pathways of ulcerative colitis. Dr. Rubin highlighted the unmet needs in current therapies and emphasized the potential of obefazimod in addressing refractory cases of the disease. With UC rising globally, particularly in regions like Asia, Dr. Rubin framed this new therapy as a necessary addition to the medical toolbox.
As a first-in-class treatment, obefazimod stands out for its unique mechanism of action. Traditional therapies target active inflammatory processes, such as cytokine activity, but obefazimod works at a different level.
“This is resetting the inflammatory cascade,” Dr. Rubin explained, likening the process to “shutting off the faucet at the sink,” rather than managing already circulating inflammatory factors.
The therapy achieves this by enhancing the expression of microRNA-124, which modulates the immune response by downregulating key proteins associated with inflammation, such as IL-6 and TNF-alpha.
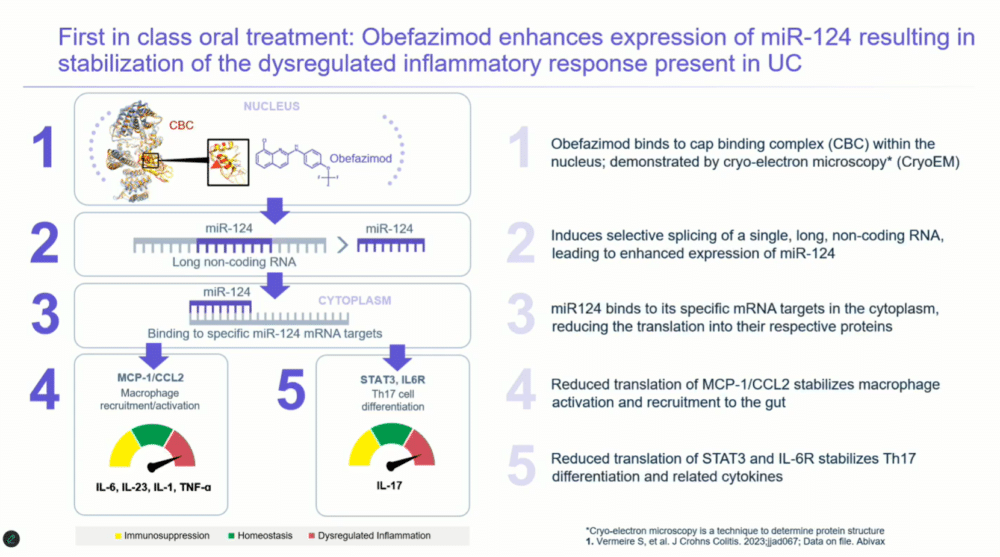
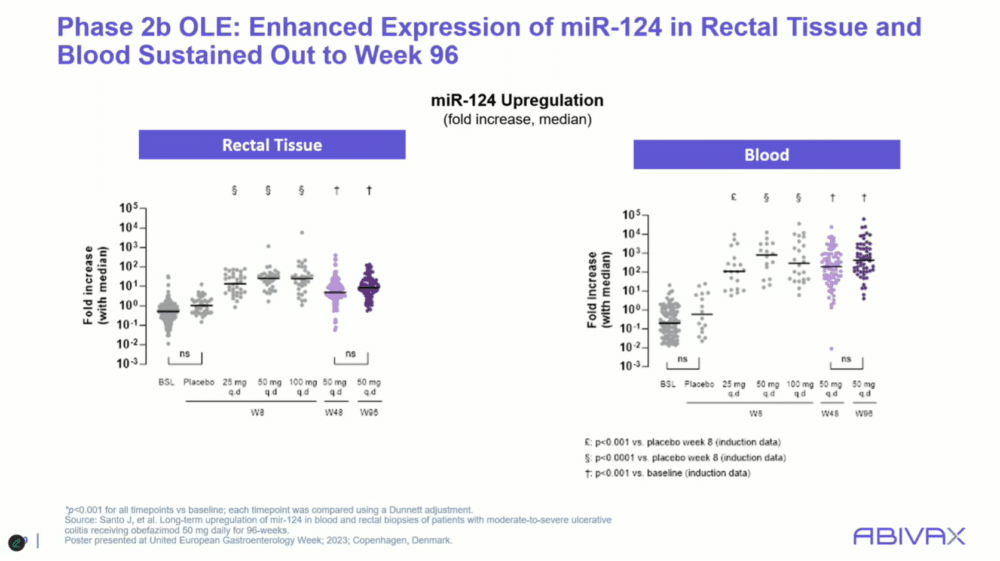
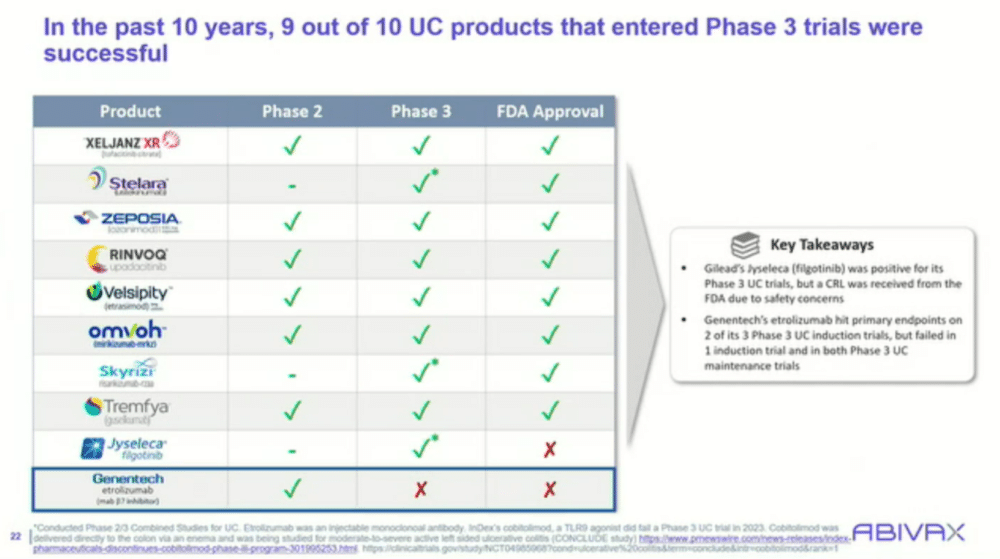
Images: Screenshot of presentation during the webinar by Abivax on ulcerative colitis treatment. Courtesy of Abivax.
Addressing Unmet Needs in Ulcerative Colitis
Ulcerative colitis is a chronic, relapsing condition that causes inflammation in the large intestine and often results in severe symptoms like rectal bleeding, diarrhea, and an urgent need to defecate. Despite significant advances in treatment options, many patients remain refractory to existing therapies. These patients are often those who have already undergone multiple treatment regimens, including biologics like anti-TNF agents (infliximab, adalimumab) or JAK inhibitors (tofacitinib). Dr. Rubin pointed out that despite the diversity of available treatments, a therapeutic ceiling exists.
“The y-axis doesn’t even go to a hundred percent,” he said, referring to the limited efficacy of current therapies in achieving full remission.
Current therapies work primarily by inhibiting key inflammatory proteins or blocking cytokine pathways involved in UC, but they often fail to fully suppress the inflammatory response. This leaves a significant portion of the patient population with uncontrolled disease, leading to poor quality of life and, in severe cases, the need for surgery.
“We’ve yet to identify the cause of colitis or medical cures,” Dr. Rubin emphasized.
This unmet need has driven the search for novel approaches like obefazimod, which does not directly target these inflammatory proteins but instead modulates upstream processes involved in the immune response.
Obefazimod’s innovative mechanism focuses on enhancing microRNA-124 expression, which stabilizes the dysregulated immune response seen in UC. By upregulating microRNA-124, the therapy affects several key inflammatory proteins involved in macrophage recruitment and T-cell differentiation. Dr. Rubin explained that microRNA-124 reduces the production of MCP1 and CCL2, as well as STAT3 and the IL-6 receptor—pathways known to drive chronic inflammation. The result is a downregulation of multiple pro-inflammatory cytokines, including IL-1, IL-6, IL-23, TNF-alpha, and IL-17. This broad-spectrum effect on inflammation is what sets obefazimod apart from current therapies.
Trial Insights and Promising Results
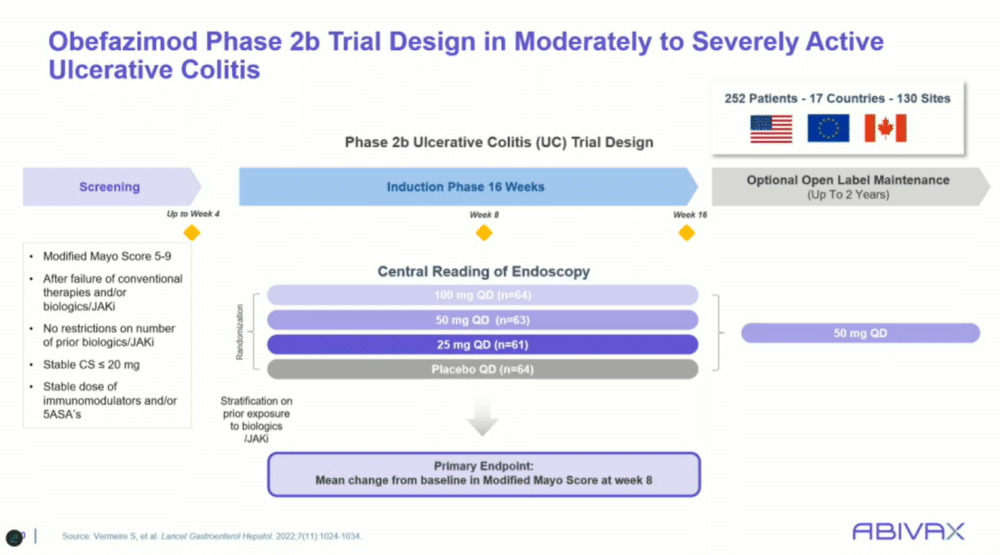
The ongoing phase 3 trial (ABTECT) of obefazimod aims to build on the encouraging results from earlier studies. In the phase 2b trial, the therapy demonstrated statistically significant improvements in both clinical remission and endoscopic healing at eight weeks, even among patients who had failed other advanced therapies.
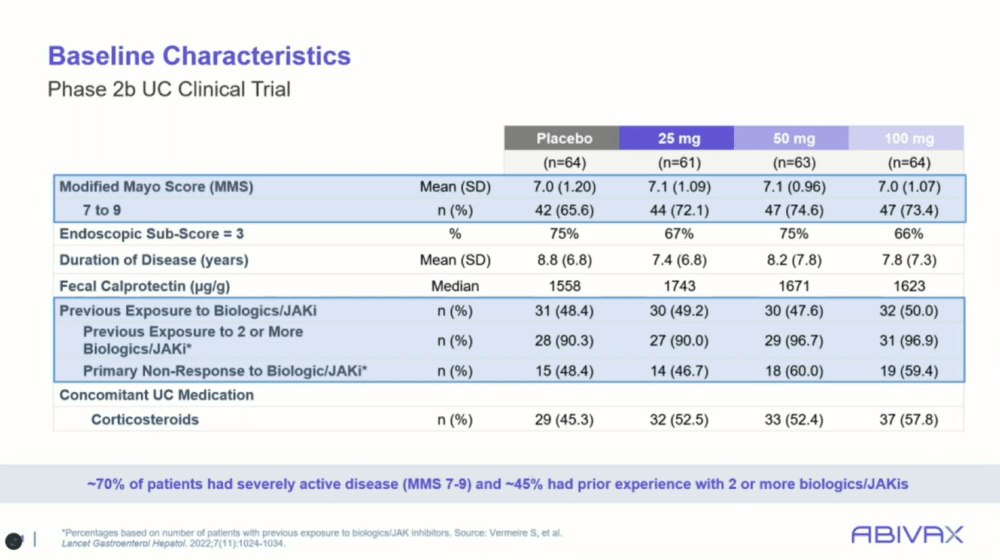
“These were sick patients with high modified Mayo scores,” said Dr. Rubin, noting that about 50% of the trial participants had prior exposure to biologics or JAK inhibitors.
This trial design, which allowed for patients with severe disease and a long history of treatment failure, highlighted obefazimod’s potential in addressing the hardest-to-treat cases.
One key feature of the phase 2b trial was its inclusion of patients who had already tried multiple advanced therapies. More than half of the participants had been on two or more biologic therapies prior to entering the study, and around 50% were also taking steroids. Despite these challenges, obefazimod showed marked improvements over placebo across all dosage groups.
“The drug worked in all three doses, even in these refractory patients,” Dr. Rubin explained, emphasizing how the therapy managed to overcome the difficult clinical profile of the participants.
In the trial, patients were randomized to receive one of three doses of obefazimod or placebo. The primary endpoint was the change in the modified Mayo score, which includes measures of stool frequency, rectal bleeding, and endoscopic findings. At eight weeks, all three doses of obefazimod demonstrated superior efficacy compared to placebo. Secondary endpoints, such as clinical remission and endoscopic improvement, also favored the active treatment. Dr. Rubin highlighted the significance of these findings:
“You see a very strong response in those treated earlier, but even in the most refractory patients, there was a significant effect.”

Moving Forward: Phase 3 and Safety
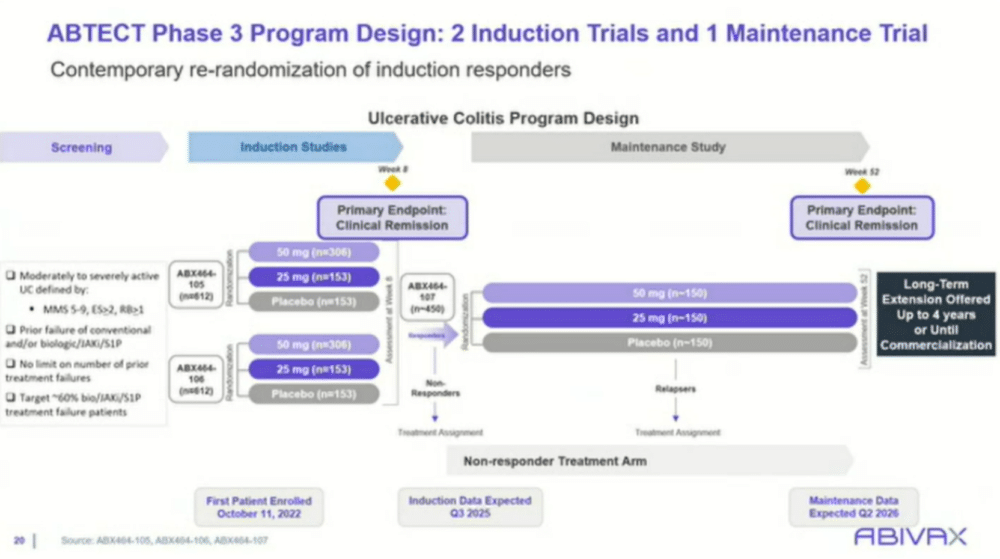
The ABTECT phase 3 trial, currently underway, aims to further evaluate obefazimod’s efficacy in a larger patient population. The trial has expanded to over 17 countries, including diverse patient demographics to better understand its global applicability. The trial design includes two induction studies (studies 105 and 106), each comparing two doses of obefazimod (25 mg and 50 mg) against placebo, with responders moving into a maintenance phase that extends for up to 52 weeks. The primary endpoint for the induction phase is clinical remission at eight weeks, with the long-term endpoint focused on maintaining remission through 52 weeks.
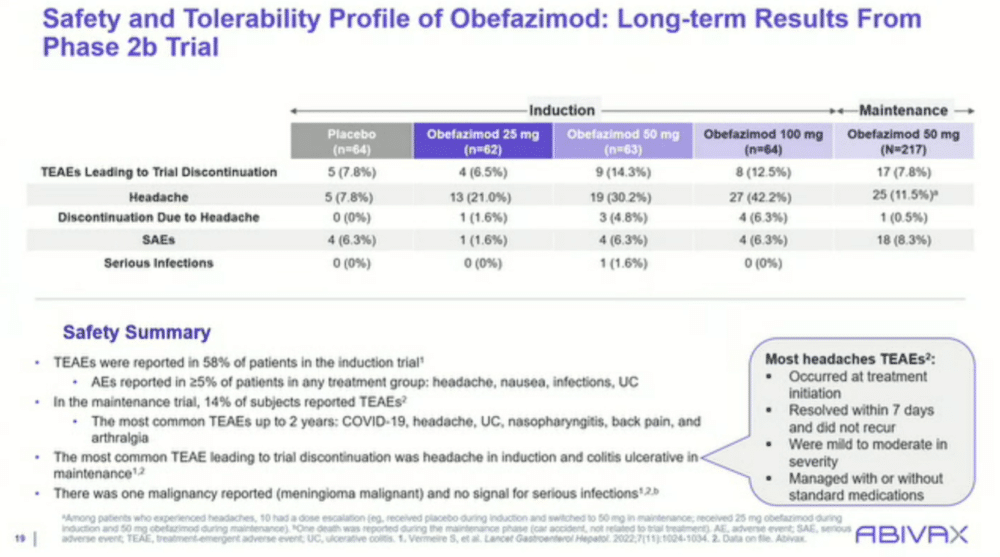
Dr. Rubin is optimistic about the future of obefazimod, not just for its efficacy but also for its safety profile. He highlighted that safety data from earlier trials indicated no serious infections, a key concern with immune-modifying therapies.
“The safety with this drug is quite good,” Rubin noted, adding that the most common side effect, headaches, was generally mild, transient, and resolved within seven days.
This favorable safety profile, coupled with the therapy’s novel mechanism, makes obefazimod an attractive option for early intervention in UC management.
Looking ahead, Dr. Rubin believes obefazimod could fill a critical gap in UC treatment. The fact that it is an oral therapy, unlike many of the current biologics that require infusion or injection, adds convenience and may appeal to both clinicians and patients. Additionally, its mechanism, which is not dependent on immunosuppression, could make it a preferred option, particularly in patients with a higher risk of infections or other complications from long-term immune suppression.
“I expect this is going to be something people want to use early,” Dr. Rubin concluded, emphasizing his belief that obefazimod will play a vital role in the future of UC treatment.
A New Horizon in Ulcerative Colitis Treatment
As the phase 3 results for obefazimod are eagerly awaited, the medical community is cautiously optimistic about its potential impact. Dr. Rubin and his colleagues, who are deeply involved in the treatment of ulcerative colitis, are enthusiastic about the therapy’s ability to reset the inflammatory process at its core. With its promising safety profile, ease of administration, and broad efficacy, obefazimod could represent a significant advancement in the management of UC, particularly for patients who have failed other therapies.
In a field where many treatments offer incremental improvements, obefazimod’s novel approach offers hope for more substantial, long-lasting benefits.
“This is a growing market with a growing need,” Dr. Rubin stated, underlining the importance of bringing effective, first-in-class therapies like obefazimod to the forefront.
If the phase 3 results continue to demonstrate positive outcomes, obefazimod could become an important tool for clinicians managing ulcerative colitis, offering a new option for refractory and newly diagnosed patients alike.